��Ŀ����
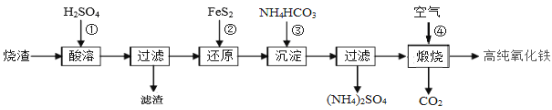
����Ŀ���Լ��ұ���һ����Ҫ���л�ԭ�ϣ��ø����ʿɺϳ��������ʡ�
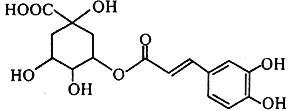
��֪����1mol D�������Ʒ�Ӧ������1mol ������
��D��E��Fÿ�ַ����о���ֻ����һ�ֺ��������š�
��G��һ���л��߷��ӻ����
�ش��������⣺
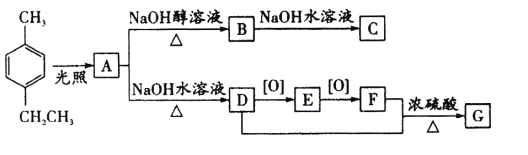
��1��A�Ľṹ��ʽΪ_______________��A��B�ķ�Ӧ����_________________��
C�й����ŵ�����_____________________��
��2��1 mol E��������������Һ��Ӧ�����ɳ��������ʵ���Ϊ_________________��
��3��D+F��G�Ļ�ѧ����ʽΪ_________________________��
��4��H��D����Է�������С14��H��ͬ���칹����ͬʱ��������������______�֡�
�ٱ�����������ȡ����;
�����Ȼ�����Һ����ɫ;
��5��д���������Һ��С�CH2Br����B��˳ʽͬ���칹��Ľṹ��ʽ___________��
���𰸡�  ��ȥ��Ӧ �ǻ���̼̼˫�� 4 mol
��ȥ��Ӧ �ǻ���̼̼˫�� 4 mol 
![]()
![]() ����
����
 12
12 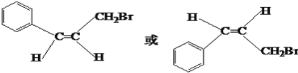
��������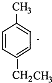 ��Һ���ڹ�����������ȡ���ڲ����ϣ�����D��E��F����������������֪�����Ǵ�ȩ��֮���ת����������֪(1)��֪ÿ��D�к��������ǻ����ٸ�����֪(2)��֪��ֻ��ȡ����
��Һ���ڹ�����������ȡ���ڲ����ϣ�����D��E��F����������������֪�����Ǵ�ȩ��֮���ת����������֪(1)��֪ÿ��D�к��������ǻ����ٸ�����֪(2)��֪��ֻ��ȡ���� ���������ϣ��õ�A(
���������ϣ��õ�A(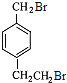 )���ٸ����л���ͼ�е����������Ƶ�DΪ��
)���ٸ����л���ͼ�е����������Ƶ�DΪ��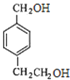 EΪ��
E��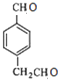 F��
FΪ��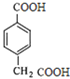 ��G��һ���л��߷��ӻ��������GΪ��
��G��һ���л��߷��ӻ��������GΪ��![]() ������A��B��C��������NaOH�Ĵ���Һ������ȥ��Ӧ��NaOH��ˮ��Һ����ȡ����Ӧ������֪BΪ
������A��B��C��������NaOH�Ĵ���Һ������ȥ��Ӧ��NaOH��ˮ��Һ����ȡ����Ӧ������֪BΪ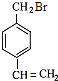 �� CΪ
�� CΪ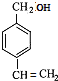 ��
��
(1)A�Ľṹ��ʽΪ ��A��BΪ±������NaOH�Ĵ���Һ������ȥ��Ӧ��
��A��BΪ±������NaOH�Ĵ���Һ������ȥ��Ӧ��
CΪ �����й����ŵ������ǻ���̼̼˫����
�����й����ŵ������ǻ���̼̼˫����
(2)���ݷ�Ӧ�� +4[Ag(NH3)2]++4OH-
+4[Ag(NH3)2]++4OH-![]()
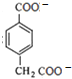 +2NH4++4Ag��+6NH3+2H2O���м���ɵ�Ag�����ʵ���Ϊ4mol��
+2NH4++4Ag��+6NH3+2H2O���м���ɵ�Ag�����ʵ���Ϊ4mol��
(3)����ǰ��ķ����ɵ�DΪ�� FΪ��
FΪ�� ��D+F��G�Ļ�ѧ����ʽΪ��
��D+F��G�Ļ�ѧ����ʽΪ��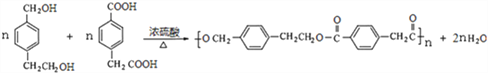 ��
��
(4)H��D��Է�������С14����֪H����ɱ�D��һ��̼�����Ȼ�����Һ����ɫ˵��H���б��ӵĽṹ��������������ȡ������˵��H������ȡ�����ڱ��������ڡ��估��λ��������������ǻ�����һ��ȡ������������CH2CH2OH����OCH2CH3����CH
(5)���������Һ�����CH2Br���� ��˳ʽͬ���칹��Ľṹ��ʽΪ
��˳ʽͬ���칹��Ľṹ��ʽΪ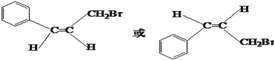 ��
��

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�