��Ŀ����
����Ŀ�����ǻ��ʹ�ҵ�ͻ����л���������Ҫԭ�ϡ�
��1���ϳɰ���Ӧ�������й����ʵĻ�ѧ�������������±���ʾ��
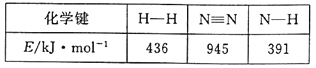
��д���úϳɰ���Ӧ���Ȼ�ѧ����ʽ___��
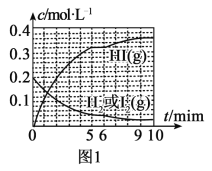
��2��һ���¶��£��ϳɰ���Ӧ��a��b���������·ֱ�ﵽƽ�⣬H2��Ũ����ʱ��ı仯��ͼ��ʾ��
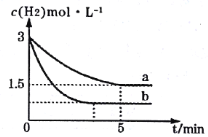
��a�����£�0��5min�ڵ�ƽ����Ӧ����v(N2)=___mol��L-1��min-1��
�����a���ԣ�b���ܸı��������___��
��3��ij��ѧ��ȤС����һ�����ܱ������г���4molN2��12molH2ģ��ϳɰ���Ӧ��ƽ�������а����������������ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ��
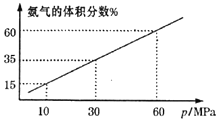
����ϵ��60MPa�´ﵽƽ�⡣H2��ƽ���ѹΪ___MPa��(��ѹ=��ѹ�����ʵ�������)����ʽ������ʱ��ƽ�ⳣ��Kp=___��(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬�������2λ��Ч����)
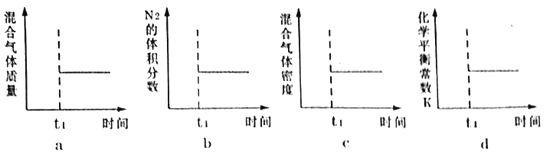
��4������ͼʾ���ܱ�ʾ�ϳɰ���Ӧ�ں��¡������ܱ���������t1ʱ���Ѿ��ﵽƽ��״̬����___��
���𰸡�N2(g)+3H2(g)![]() 2NH3(g)��H=-93kJ��mol-1 0.1mol��L-1��min-1 ����N2Ũ�� 18 0.037 b
2NH3(g)��H=-93kJ��mol-1 0.1mol��L-1��min-1 ����N2Ũ�� 18 0.037 b
��������
(1)�ɼ��������ȣ�945 kJ +3��436 kJ=2253kJ���½��ϳɷ��ȣ�6��391 kJ=2346kJ�����ȶ࣬��ѧ��Ӧ����Ϊ���ȣ��ų�����93kJ���Ȼ�ѧ����ʽΪN2(g)+3H2(g)![]() 2NH3(g)��H=-93kJ��mol-1��
2NH3(g)��H=-93kJ��mol-1��
��2��a�����£�0��5min�ڵ�ƽ����Ӧ����v(H2)=![]() ������ϵ���ȿ�֪v(N2)=0.1mol��L-1��min-1��
������ϵ���ȿ�֪v(N2)=0.1mol��L-1��min-1��
a��b������ʼŨ����ͬ��b����ƽ���ʱ�����̣�˵����Ӧ��������ƽ��ʱ������Ũ�ȼ�С��˵��ƽ�������ƶ������Ըı������������c��N2����
��3��������ϵ��60MPa�´ﵽƽ�⣬��ͬ�¶��£����������������������ʵ����ķ�������
ƽ��ʱ�����������=![]()
���x=3
H2��ƽ���ѹ=![]()
��������ƽ�����ݼ���Kp![]()
��4��a.�������ʼ�����������غ㣬�����������䲻��ƽ��ı�־��
b.N2�����������˵���Ѿ�ƽ�⣻
c.�������ʼ�����������غ㣬������������ܶȲ��䲻��ƽ��ı�־��
d.��ѧƽ�ⳣ��ֻ���¶��йأ�����ƽ��ı�־��
��ѡb��

 ��У����ϵ�д�
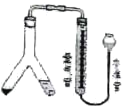
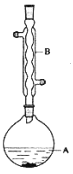
��У����ϵ�д�����Ŀ������������������۷���Ϣ���ʵijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧװ��ʾ��ͼ���й��������£�
![]() +H2O
+H2O
���ԭ������ | �ܶ�/��g��cm-3�� | �е�/�� | ˮ���ܽ��� | |
���촼 | 88 | 0.8123 | 131 | �� |
���� | 60 | 1.0492 | 118 | �� |
���������� | 130 | 0.8670 | 142 | ���� |
ʵ�鲽�裺
��A�м���4.4g�����촼��6.0g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50���ӣ���ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮ����þ���壬����Ƭ�̣����˳�ȥ����þ���壬�������������ռ�140��143����֣�������������3.9g���ش��������⣺
��1��װ��B�������ǣ�___��
��2���÷�Ӧ��Ũ���������___������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ___��
��3���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ����___��
��4����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ���ǣ�___���ڶ���ˮϴ����ҪĿ���ǣ�___��
��5����ʵ���м�����������Ŀ���ǣ�___��
��6������������У�����ѡ��װ����ȷ���ǣ�___(����)
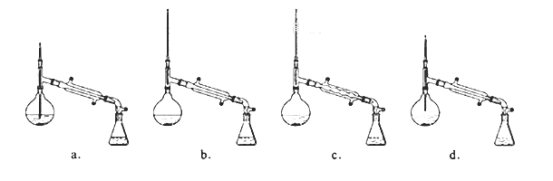
��7����ʵ��IJ�����___��
A.30�G B.40�G C.50�G D.60�G
��8���ڽ����������ʱ������130�濪ʼ�ռ���֣�����ƫ___(����ߵ�)ԭ����___��