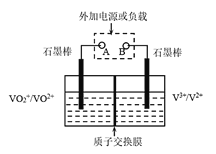
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΒβΜ·«β≥Θ”Ο”Ύ÷Τ±ΗΒβΒΡΜ·ΚœΈοΚΆ”ΟΉς”–ΜζΖ¥”ΠΒΡΜΙ‘≠ΦΝΓΘΒβΜ·«β≤ΜΈ»Ε®Θ§“ΉΖ÷ΫβΘ§ΒβΜ·«βΦΪ“Ή»ή”ΎΥ°Θ§ΤδΥ°»ή“Κ≥ΤΈΣ«βΒβΥαΘ§«βΒβΥα ««ΩΥαΘ§”–Ϋœ«ΩΒΡΜΙ‘≠–‘ΓΘ
(1)«βΒβΥα»τ‘ΎΩ’Τχ÷–≥ΛΤΎΖ≈÷ΟΘ§»ή“ΚΜα±δ≥…ΜΤ…ΪΘ§Τδ‘≠“ρ «___Θ®”ΟΜ·―ßΖΫ≥Χ Ϋά¥±μ ΨΘ©ΓΘ
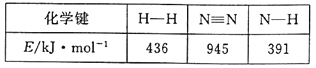
(2) Β―ι “÷–≥Θ”ΟΗ…‘οΒΡΚλΝΉΚΆΒβœύΜΞΫ”¥ΞΘ§Φ”…ΌΝΩΥ°ΈΔ»»Θ§Φ¥Ω……ζ≥…ΒβΜ·«βΚΆ―«ΝΉΥαΘ®H3PO3Θ©Θ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ___ΓΘ
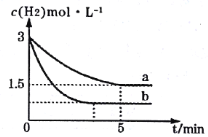
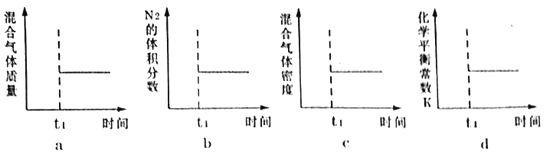
(3)«βΤχΚΆΒβ’τΤχΡή÷±Ϋ”Ζ¥”Π…ζ≥…ΒβΜ·«βΘ§H2(g)ΘΪI2(g)![]() 2HI(g) ΠΛHΘΦ0ΓΘTΓφ ±Θ§œρ1LΚψ»ίΟή±’»ίΤς÷–≥δ»κ0.2molH2ΚΆ0.2molI2(g)Θ§5min ±Ζ¥”Π¥οΒΫΤΫΚβΘ§H2ΓΔI2(g)ΚΆHIΒΡΈο÷ ΒΡΝΩ≈®Ε»(c)Υφ ±Φδ(t)±δΜ·ΒΡ«ζœΏ»γΆΦlΥυ ΨΘΚ
2HI(g) ΠΛHΘΦ0ΓΘTΓφ ±Θ§œρ1LΚψ»ίΟή±’»ίΤς÷–≥δ»κ0.2molH2ΚΆ0.2molI2(g)Θ§5min ±Ζ¥”Π¥οΒΫΤΫΚβΘ§H2ΓΔI2(g)ΚΆHIΒΡΈο÷ ΒΡΝΩ≈®Ε»(c)Υφ ±Φδ(t)±δΜ·ΒΡ«ζœΏ»γΆΦlΥυ ΨΘΚ
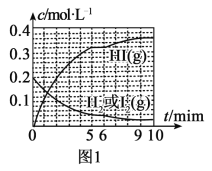
ΔΌ0ΓΪ5minΡΎΘ§“‘HI±μ ΨΒΡΗΟΖ¥”ΠΥΌ¬ v(HI)=___ΓΘ
ΔΎTΓφ ±Θ§ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=___ΓΘ
Δέ6min ±Θ§ΗΡ±δΒΡΆβΫγΧθΦΰΈΣ___ΓΘ
Δή10min ±Θ§±Θ≥÷ΤδΥϊΧθΦΰ≤Μ±δΘ§‘Όœρ»ίΤς÷–≥δ»κ0.1molH2ΓΔ0.1molI2(g)ΓΔ0.2molHI(g)Θ§12min ±¥οΒΫ–¬ΤΫΚβΓΘ‘ΎΆΦ2÷–Μ≠≥ω10ΓΪ12minΘ§H2ΚΆHIΒΡ≈®Ε»±δΜ·«ζœΏ___Θ®«ζœΏ…œ±ξΟςH2ΚΆHIΘ©ΘΜ0ΓΪ5minΚΆ0ΓΪ2min ±ΦδΕΈΘ§H2ΒΡΉΣΜ·¬ Ζ÷±π”ΟΠΝ1ΓΔΠΝ2±μ ΨΘ§‘ρΠΝl___ΠΝ2Θ®ΧνΓΑΘΨΓ±ΓΔΓΑΘΦΓ±ΜρΓΑ=Γ±Θ©ΓΘ
ΓΨ¥πΑΗΓΩ4HIΘΪO2=2H2OΘΪ2I2 2PΘΪ3I2ΘΪ6H2O=2H3PO3ΘΪ6HI 0.064molΓΛL-1ΓΛmin-1 64 ΫΒΈ¬ 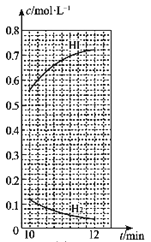 ΘΦ
ΘΦ
ΓΨΫβΈωΓΩ
(1)«βΒβΥαΨΏ”–Ϋœ«ΩΒΡΜΙ‘≠–‘Θ§¬Ε÷Ο‘ΎΩ’Τχ÷–Μα±ΜΩ’Τχ÷–ΒΡ―θΤχ―θΜ·ΈΣΒβΒΞ÷ Θ§¥”Εχ Ι»ή“Κ±δΜΤΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ4HIΘΪO2=2H2OΘΪ2I2ΓΘ¥πΑΗΈΣΘΚ4HIΘΪO2=2H2OΘΪ2I2ΘΜ
(2)ΗυΨίΧβ“βΚΆ―θΜ·ΜΙ‘≠Ζ¥”ΠΖΫ≥Χ ΫΒΡ≈δΤΫ‘≠‘ρΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ2PΘΪ3I2ΘΪ6H2O=2H3PO3ΘΪ6HIΓΘ¥πΑΗΈΣΘΚ2PΘΪ3I2ΘΪ6H2O=2H3PO3ΘΪ6HIΘΜ
(3)ΔΌ”…ΆΦœώΩ…÷ΣΘ§0ΓΪ5minΡΎΘ§ΠΛc(HI)=0.32molΓΛL-1Θ§v(HI)=![]() =0.064molΓΛL-1ΓΛmin-1ΓΘ¥πΑΗΈΣΘΚ0.064molΓΛL-1ΓΛmin-1ΘΜ
=0.064molΓΛL-1ΓΛmin-1ΓΘ¥πΑΗΈΣΘΚ0.064molΓΛL-1ΓΛmin-1ΘΜ
ΔΎTΓφ ±Θ§ΗΟΖ¥”Π‘Ύ5min ±¥οΒΫΤΫΚβΘ§”…ΆΦœώΩ…÷ΣΘ§ΤΫΚβ ±Θ§c(HI)=0.32molΓΛL-1Θ§c(H2)=c(I2)=0.04molΓΛL-1Θ§‘ρTΓφ ±ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=![]() =
=![]() =64ΓΘ¥πΑΗΈΣΘΚ64ΘΜ
=64ΓΘ¥πΑΗΈΣΘΚ64ΘΜ
Δέ6min ±Θ§Ζ¥”ΠΈοΓΔ≤ζΈο≈®Ε»Υ≤Φδ≤Μ±δΘ§ΒΪΥφΉ≈Ζ¥”ΠΒΡΫχ––Θ§Ζ¥”ΠΈο≈®Ε»Φθ…ΌΘ§…ζ≥…Έο≈®Ε»‘ωΦ”Θ§ΥΒΟςΤΫΚβ’ΐœρ“ΤΕ·Θ§”…”ΎΗΟ’ΐΖ¥”ΠΈΣΖ≈»»Ζ¥”ΠΘ§Ι 6min ±ΗΡ±δΒΡΧθΦΰ «ΫΒΒΆΈ¬Ε»ΓΘ¥πΑΗΈΣΘΚΫΒΈ¬ΘΜ
Δή10min ±Θ§“―¥οΒΫ–¬ΒΡΤΫΚβΘ§¥Υ ±c(H2)=c(I2)=0.02molΓΛL-1Θ§c(HI)=0.36molΓΛL-1Θ§‘ρK=![]() =
=![]() =324ΓΘ±Θ≥÷ΤδΥϊΧθΦΰ≤Μ±δΘ§‘Όœρ»ίΤς÷–≥δ»κ0.1molH2ΓΔ0.1molI2(g)ΓΔ0.2molHIΘ§¥Υ ±Θ§c(H2)=c(I2)=0.12molΓΛL-1Θ§c(HI)=0.56molΓΛL-1Θ§Qc=
=324ΓΘ±Θ≥÷ΤδΥϊΧθΦΰ≤Μ±δΘ§‘Όœρ»ίΤς÷–≥δ»κ0.1molH2ΓΔ0.1molI2(g)ΓΔ0.2molHIΘ§¥Υ ±Θ§c(H2)=c(I2)=0.12molΓΛL-1Θ§c(HI)=0.56molΓΛL-1Θ§Qc=![]() =
=![]() =
=![]() ΘΦK=324Θ§ΤΫΚβ’ΐœρ“ΤΕ·Θ§c(H2)ΚΆc(I2)Φθ–ΓΘ§c(HI)‘ω¥σΘ§12min ±Θ§Ζ¥”Π¥οΒΫ–¬ΤΫΚβΘ§…η10minΓΪ12minΙΐ≥Χ÷–ΠΛc(H2)=ΠΛc(I2)=xmolΓΛL-1Θ§‘ρ12min ±Θ§c(H2)=c(I2)=(0.12Θ≠x)molΓΛL-1Θ§c(HI)=(0.56ΘΪ2x)molΓΛL-1Θ§‘ρK=
ΘΦK=324Θ§ΤΫΚβ’ΐœρ“ΤΕ·Θ§c(H2)ΚΆc(I2)Φθ–ΓΘ§c(HI)‘ω¥σΘ§12min ±Θ§Ζ¥”Π¥οΒΫ–¬ΤΫΚβΘ§…η10minΓΪ12minΙΐ≥Χ÷–ΠΛc(H2)=ΠΛc(I2)=xmolΓΛL-1Θ§‘ρ12min ±Θ§c(H2)=c(I2)=(0.12Θ≠x)molΓΛL-1Θ§c(HI)=(0.56ΘΪ2x)molΓΛL-1Θ§‘ρK=![]() =
=![]() =324Θ§ΫβΒΟΘΚx=0.08Θ§Ι 12min ±Θ§c(H2)=c(I2)=(0.12Θ≠0.08)molΓΛL-1=0.04molΓΛL-1Θ§c(HI)=(0.56ΘΪ0.16)molΓΛL-1=0.72molΓΛL-1Θ§Ι 10ΓΪ12minΘ§H2ΚΆHIΒΡ≈®Ε»±δΜ·«ζœΏ»γΆΦ
=324Θ§ΫβΒΟΘΚx=0.08Θ§Ι 12min ±Θ§c(H2)=c(I2)=(0.12Θ≠0.08)molΓΛL-1=0.04molΓΛL-1Θ§c(HI)=(0.56ΘΪ0.16)molΓΛL-1=0.72molΓΛL-1Θ§Ι 10ΓΪ12minΘ§H2ΚΆHIΒΡ≈®Ε»±δΜ·«ζœΏ»γΆΦ Υυ ΨΓΘ
Υυ ΨΓΘ
”…ΆΦ ΨΩ…÷ΣΘ§0ΓΪ5min ±Θ§H2ΒΡΉΣΜ·¬ ΠΝ1=![]() ΓΝ100%=80%Θ§0ΓΪ12min ±Θ§Ι≤≥δ»κΒΡn(H2)=0.3molΘ§12min ±Θ§ Θ”ύn(H2)=0.04molΓΛL-1ΓΝ1L=0.04molΘ§‘ρ0ΓΪ12min ±Θ§H2ΒΡΉΣΜ·¬ ΠΝ2=
ΓΝ100%=80%Θ§0ΓΪ12min ±Θ§Ι≤≥δ»κΒΡn(H2)=0.3molΘ§12min ±Θ§ Θ”ύn(H2)=0.04molΓΛL-1ΓΝ1L=0.04molΘ§‘ρ0ΓΪ12min ±Θ§H2ΒΡΉΣΜ·¬ ΠΝ2=![]() ΓΝ100%Γ÷86.7%Θ§Ι ΠΝ1ΘΦΠΝ2ΓΘ¥πΑΗΈΣΘΚ
ΓΝ100%Γ÷86.7%Θ§Ι ΠΝ1ΘΦΠΝ2ΓΘ¥πΑΗΈΣΘΚ ΘΜΘΦΓΘ
ΘΜΘΦΓΘ

 ΡήΝΠΤάΦέœΒΝ–¥πΑΗ
ΡήΝΠΤάΦέœΒΝ–¥πΑΗ ΧΤ”ΓΈΡΜ·ΩΈ ±≤βΤάœΒΝ–¥πΑΗ
ΧΤ”ΓΈΡΜ·ΩΈ ±≤βΤάœΒΝ–¥πΑΗ ΒΦ―ß”κ≤β ‘œΒΝ–¥πΑΗ
ΒΦ―ß”κ≤β ‘œΒΝ–¥πΑΗΓΨΧβΡΩΓΩœ¬±μ «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷Θ§Ε‘”Ύ±μ÷–‘ΣΥΊΔΌΓΪΔύΘ§ΧνΩ’ΜΊ¥πΘΚ
Ήε ÷ήΤΎ | ΔώA | ΔρA | ΔσA | ΔτA | ΔθA | ΔωA | ΔθΔρA | 0 |
Εΰ | ΔΌ | ΔΎ | Δέ | Δή | ||||
»ΐ | Δί | Δό | ΔΏ | Δύ |
(1)ΒΊΩ«÷–Κ§ΝΩΉνΕύΒΡ‘ΣΥΊ «______Θ§Ζ«Ϋπ τ–‘Ήν«ΩΒΡ‘ΣΥΊ «______ΓΘ
(2)–¥≥ωΔΌΒΡΉνΦρΒΞΒΡΤχΧ§«βΜ·ΈοΒΡΒγΉ” Ϋ______ΓΘ
(3)‘ΎΒΎ»ΐ÷ήΤΎ÷ςΉε‘ΣΥΊ÷–Θ§ΒΞ÷ ―θΜ·–‘Ήν«ΩΒΡ «_____Θ§Ρή–Έ≥…ΒΡΕΰ‘Σ«ΩΥα «________ΓΘ
(4)–¥≥ωΔΎΒΡΤχΧ§«βΜ·Έο”κΔΎΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_____ΓΘ
ΓΨΧβΡΩΓΩΗΏ¥ΩΝρΥαΟΧΉςΈΣΚœ≥…ΡχνήΟΧ»ΐ‘Σ’ΐΦΪ≤ΡΝœΒΡ‘≠ΝœΘ§ΙΛ“Β…œΩ…”…Χλ»ΜΕΰ―θΜ·ΟΧΖέ”κΝρΜ·ΟΧΩσΘ®ΜΙΚ§FeΓΔAlΓΔMgΓΔZnΓΔNiΓΔSiΒ»‘ΣΥΊΘ©÷Τ±ΗΘ§ΙΛ“’»γœ¬ΆΦΥυ ΨΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
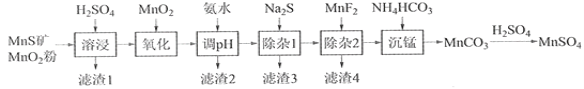
œύΙΊΫπ τάκΉ”[c0(Mn+)=0.1 molΓΛL1]–Έ≥…«β―θΜ·Έο≥ΝΒμΒΡpHΖΕΈß»γœ¬ΘΚ
Ϋπ τάκΉ” | Mn2+ | Fe2+ | Fe3+ | Al3+ | Mg2+ | Zn2+ | Ni2+ |
ΩΣ Φ≥ΝΒμΒΡpH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 | 6.2 | 6.9 |
≥ΝΒμΆξ»ΪΒΡpH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 | 8.2 | 8.9 |
Θ®1Θ©ΓΑ¬Υ‘ϋ1Γ±Κ§”–SΚΆ__________________________ΘΜ–¥≥ωΓΑ»ήΫΰΓ±÷–Εΰ―θΜ·ΟΧ”κΝρΜ·ΟΧΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_______________________________________ΓΘ
Θ®2Θ©ΓΑ―θΜ·Γ±÷–ΧμΦ” ΝΩΒΡMnO2ΒΡΉς”Ο «ΫΪ________________________Θ§Φλ―ι…ζ≥…άκΉ” ‘ΦΝ___________ΓΘ
Θ®3Θ©ΓΑΒςpHΓ±≥ΐΧζΚΆ¬ΝΘ§»ή“ΚΒΡpHΖΕΈß”ΠΒςΫΎΈΣ_______ΓΪ6÷°ΦδΘ§≥ΐ¬ΝΒΡάκΉ”Ζ¥”ΠΖΫ≥Χ Ϋ «__________ΓΘ
Θ®4Θ©ΓΑ≥ΐ‘”1Γ±ΒΡΡΩΒΡ «≥ΐ»ΞZn2+ΚΆNi2+Θ§ΓΑ¬Υ‘ϋ3Γ±ΒΡ÷ς“Σ≥…Ζ÷ «______________ΓΘ
Θ®5Θ©―θΜ·÷–≥ΐΝΥΧμΦ” ΝΩMnO2ΜΙΩ…“‘”ΟH2O2ά¥―θΜ·Θ§–¥≥ωΗΟάκΉ”Ζ¥”Π_______________ΓΘ
Θ®6Θ©–¥≥ωΓΑ≥ΝΟΧΓ±ΒΡάκΉ”ΖΫ≥Χ Ϋ_______________________________________ΓΘ
Θ®7Θ©≤ψΉ¥ΡχνήΟΧ»ΐ‘Σ≤ΡΝœΩ…ΉςΈΣο°άκΉ”Βγ≥Ί’ΐΦΪ≤ΡΝœΘ§ΤδΜ·―ß ΫΈΣLiNixCoyMnzO2Θ§Τδ÷–NiΓΔCoΓΔMnΒΡΜ·ΚœΦέΖ÷±πΈΣ+2ΓΔ+3ΓΔ+4ΓΘΒ±x=y=![]() ±Θ§z=___________ΓΘ
±Θ§z=___________ΓΘ
Θ®8Θ©–¥≥ωFe2+ΚΆHNO3ΒΡάκΉ”Ζ¥”Π__________ΓΘ