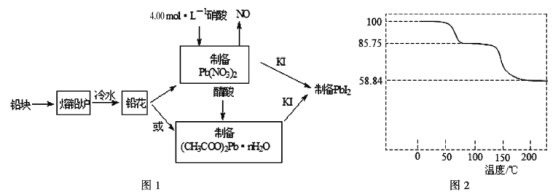
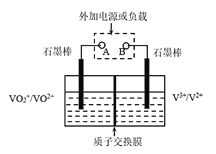
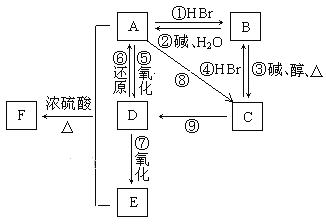
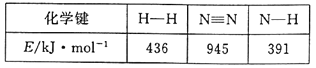
��Ŀ����
����Ŀ��ij�¶�ʱ��Ag2SO4��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ���ǣ� ��
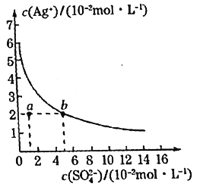
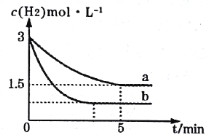
A.���д���SO42-����Һ�п϶�������Ag+
B.���¶��£�Ag2SO4���ܶȻ�����(Ksp)����������3
C.����ͨ���ı��¶Ȼ��������������ʹa���ƶ���b��
D.���¶��£�0.02mol��L-1��AgNO3��Һ��0.2mol��L-1��Na2SO4��Һ�������ϣ��������ɳ���
���𰸡�D
��������
Ag2SO4��ˮ�г����ܽ�ƽ��Ϊ��Ag2SO4��s��![]() 2Ag+��aq��+SO42-��aq����ksp=c2��Ag+����c��SO42-�������������ϵĵ�Ϊ����״̬�����������ϵĵ�Ϊ�DZ���״̬���ݴ˽��
2Ag+��aq��+SO42-��aq����ksp=c2��Ag+����c��SO42-�������������ϵĵ�Ϊ����״̬�����������ϵĵ�Ϊ�DZ���״̬���ݴ˽��
A��Ag2SO4��ˮ�г����ܽ�ƽ��Ϊ��Ag2SO4��s��![]() 2Ag+��aq��+SO42-��aq�����ܽ�Ϊ������̣����д���SO42-����Һ��һ������Ag+��A����
2Ag+��aq��+SO42-��aq�����ܽ�Ϊ������̣����д���SO42-����Һ��һ������Ag+��A����
B������ksp=c2��Ag+����c��SO42-������b�㣬��c��SO42-��=5��10-2mol/Lʱ��c��Ag+��=2��10-2mol/L��ksp=c2��Ag+����c��SO42-����2��10-5��Ag2SO4���ܶȻ�����(Ksp)����������10-5��B����
c����������ʱ����Һ�������Ӻ����������Ũ�ȶ�����C����
D������Qc= c2��Ag+����c��SO42-����10-5��ksp���������ɳ�����D��ȷ��
��ѡD

����Ŀ���±���Ԫ�����ڱ���һ���֣����ڱ���Ԫ�آ١��࣬��ջش�
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A | 0 |
�� | �� | �� | �� | �� | ||||
�� | �� | �� | �� | �� |
(1)�ؿ��к�������Ԫ����______���ǽ�������ǿ��Ԫ����______��
(2)д���ٵ������̬�⻯��ĵ���ʽ______��
(3)�ڵ�����������Ԫ���У�������������ǿ����_____�����γɵĶ�Ԫǿ����________��
(4)д���ڵ���̬�⻯����ڵ�����������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ_____��