��Ŀ����
��������(H2O2)��һ����ɫ��Һ��,����ˮ��Һ�׳�˫��ˮ,��������,����������������ɱ������Ư���ȡ�
(1)����˵����ȷ���� ��
| A��������������м��м��Լ����зǼ��Լ� |
| B��H2O2��H2O��Ϊͬ�������� |
| C��34 g H2O2�к��е���������ΪNA |
| D��ʵ���ҿ������ù���������ȡ���� |
(3)��H2O2��Һ��������FeCl2��Һ��,��Һ��dz��ɫ��Ϊ�ػ�ɫ,д���÷�Ӧ�����ӷ���ʽ: ��
(4)ij����ҵ��ˮ�к���һ��������,Ϊ�˳�ȥ����,������H2O2�����ȼ�,д���÷�Ӧ�Ļ�ѧ����ʽ: ��
(1)AD
(2)H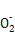

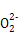 +H+
+H+
(3)H2O2+2Fe2++2H+=2Fe3++2H2O
(4)H2O2+Cl2=2HCl+O2��
����

��ϰ��ϵ�д�
�����Ŀ
�����ߡ������־���ҹ��ĺ�����ҵ�������µ�ƪ�¡�
��1���������ʱ�������������ľ���Ħ�����������¡�Ϊ�˷�ֹ����¶ȹ��ߣ��ڻ��һ��Ϳ��һ�������Ϳ�ϣ���Ϳ�ϵ���������ܵ��� ��
| A���ڸ����²��ڻ� | B���ڸ����¿ɷֽ����� |
| C���ڳ����¾ͷֽ����� | D����Ϳ�ϲ����ܷ����ֽ� |
�÷�Ӧ�б�������ԭ���뱻��ԭ��ԭ�ӵ����ʵ���֮���� �������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��� ��
��3����ͼ��ij�ռ�վ����ת��ϵͳ�ľֲ�ʾ��ͼ������ȼ�ϵ�ز���KOHΪ���Һ��ȼ�ϵ�طŵ�ʱ�ĸ�����ӦΪ�� �����ij��ʱ�������������й��ռ���33.6L���壨������ɱ��������ö�ʱ����ˮ���ϵͳ��ת�Ƶ��ӵ����ʵ���Ϊ mol��

��4�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO2=2CO+O2��CO������ȼ�ϡ� ��֪�÷�Ӧ��������ӦΪ��4OH��4e-=O2��+2H2O ��������ӦΪ�� �����������������Ʒ�Ӧ2CO=2C+O2����H��0����S��0��������CO����Ⱦ�������ж�������Ӧ�Ƿ��ܷ����� ���� ��
��5��.�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ(C3H6)����������ɵñ�ϩ��
��֪����C3H8(g)
 CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1
CH4(g)+HC��CH(g)+H2(g)�� ��H1="156.6" kJ��mol-1��CH3CH=CH2(g)
 CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1
CH4(g)+ HC��CH (g)����H2="32.4" kJ��mol-1����ͬ�����£���ӦC3H8(g)
 CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1
CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1