��Ŀ����
��1���뽫�����������ʣ�KBr��Br2��I2��KI��K2SO4�ֱ��������к����ϣ����һ��δ��ƽ�Ļ�ѧ����ʽ��
KBrO3��________��H2SO4�D��________��________��________��________��H2O��
��2������û�ѧ����ʽ��I2��KBr�Ļ�ѧ�������ֱ���8��1����
��Br2�Ļ�ѧ��������________��
���뽫��Ӧ��Ļ�ѧʽ����ƽ��Ļ�ѧ����������������Ӧ��λ���У�
________KBrO3��________��________H2SO4�D��������
����ת��10 mol���ӣ���Ӧ������I2�����ʵ���Ϊ________��
��1��KI��I2��Br2��K2SO4��KBr
��2����1����3��16KI��9����5 mol
����

��������(H2O2)��һ����ɫ��Һ��,����ˮ��Һ�׳�˫��ˮ,��������,����������������ɱ������Ư���ȡ�
(1)����˵����ȷ���� ��
| A��������������м��м��Լ����зǼ��Լ� |
| B��H2O2��H2O��Ϊͬ�������� |
| C��34 g H2O2�к��е���������ΪNA |
| D��ʵ���ҿ������ù���������ȡ���� |
(3)��H2O2��Һ��������FeCl2��Һ��,��Һ��dz��ɫ��Ϊ�ػ�ɫ,д���÷�Ӧ�����ӷ���ʽ: ��
(4)ij����ҵ��ˮ�к���һ��������,Ϊ�˳�ȥ����,������H2O2�����ȼ�,д���÷�Ӧ�Ļ�ѧ����ʽ: ��
���������ھ�������������ˮ��������������ҵ�������������.
��1���������������������ҿ������������е��ʷ�Ӧ����:6Ag(s)+O3(g)=3Ag2O(s) ��H=-235.8kJ/mol.��֪2Ag2O(s)=4Ag(s)+O2(g) ��H=+62.2kJ/mol,�����·�Ӧ: 2O3(g)=3O2(g)�Ħ�H= .
��2����ѧ��P.Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в���,�缫��ӦʽΪ3H2O-6e-=O3��+6H+,���������ܽ���ˮ�е��������ɹ�������,��缫��ӦʽΪ ��
��3��O3�ڼ��������¿ɽ�Na2SO4������Na2S2O8��д���÷�Ӧ�Ļ�ѧ����ʽΪ�� 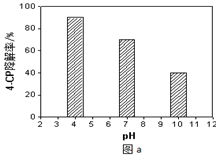

��4�����õ�Na2S2O8��Һ�ɽ����л���Ⱦ��4-CP��ԭ����Na2S2O8��Һ��һ�������¿ɲ���ǿ���������ɻ���SO4-������ͨ���ⶨ4-CP�����ʿ��ж�Na2S2O8��Һ����SO4-��������ij�о�С�����ʵ��̽������Һ����ԡ�Fe2+��Ũ�ȶԲ���SO4-����Ӱ�졣
����Һ����Ե�Ӱ�죺����������ͬ����4-CP���뵽��ͬpH��Na2S2O8��Һ�У������ͼa��ʾ���ɴ˿�֪����Һ������ǿ�� ���� �������ڡ������ڡ���Na2S2O8����SO4-����
��Fe2+Ũ�ȵ�Ӱ�죺��ͬ�����£�����ͬŨ�ȵ�FeSO4��Һ�ֱ����c(4-CP)=1.56��10-4 mol��L��1��c(Na2S2O8)=3.12��10-3 mol��L��1�Ļ����Һ�С���Ӧ240 min����ʵ������ͼb��ʾ����֪ S2O82- + Fe2+= SO4-��+ SO42- + Fe3+������ͼʾ��֪����˵����ȷ���ǣ�_________________������ţ�
| A����Ӧ��ʼһ��ʱ���ڣ� 4-CP��������Fe2+Ũ�ȵ������������ԭ����Fe2+��ʹNa2S2O8���������SO4-���� |
| B��Fe2+��4-CP���ⷴӦ�Ĵ��� |
| C����c(Fe2+)����ʱ��4-CP�����ʷ����½���ԭ�������Fe2+����SO4����������Ӧ�����IJ���SO4������ |
| D��4-CP�����ʷ����½���ԭ����������ɵ�Fe3+ˮ��ʹ��Һ��������ǿ�������ڽ��ⷴӦ�Ľ��С� |
��֪������ԭ��Ӧ�����ֻ�����Ӧ���͵Ĺ�ϵ������ͼ��ʾ����������ˮ�μӻ����ɵļ��ַ�Ӧ��
��CaO+H2O =Ca(OH)2
��2Na+H2O=2NaOH+H2��
��H2+CuO  Cu +H2O
Cu +H2O
��3S+6NaOH  2Na2S +Na2SO3 +3H2O
2Na2S +Na2SO3 +3H2O
��NaOH+HCl=NaCl+H2O
��ش��������⣺
��1����Ӧ����ˮ ������ĸ����
| A���������� |
| B���ǻ�ԭ�� |
| C���������������ǻ�ԭ�� |
| D���Ȳ����������ֲ��ǻ�ԭ�� |
��3��������Ӧ�У������������� ������ţ���
��4��д��һ�ַ���������Ҽ��г�����������ˮ���ɵ����ӷ���ʽ�� ��
