��Ŀ����
A��B��C��D��E��F�������ʵ��ת����ϵ����ͼ��ʾ����Ӧ���������ֲ����δ�г�����
��1����A��D��F�����������ķǽ������ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ���֪D�����ڵ��ӹ�ҵ������Ҫ���ã���D��ԭ�ӽṹʾ��ͼΪ ������E�ĽṹʽΪ ����Ӧ�ٵĻ�ѧ����ʽΪ__________________��
��2����A�dz����������ʣ�D����̬�ǽ������ʣ�F��Һ̬�ǽ������ʣ���Ӧ�١��ڶ�����Һ�н��У�д��A�����ڱ��е�λ�� ���õ���ʽ��ʾB���γɹ��� ������C������ʵ�����Cl2����Һ�з�Ӧ�����ӷ�Ӧ����ʽΪ ��
��3����A��DΪ�����������ʣ�A��D�ֱ���F��Ũ��Һ�ڼ��������²��ܷ�����Ӧ�� д����Ӧ�ܵĻ�ѧ����ʽ ��������A����ҺB��ַ�Ӧ��������C�н��������ӵļ��鷽��Ϊ �����Զ��Ե缫���B��ˮ��Һ������������3.2gʱ�������������������ڱ�״����Ϊ L��
(1) ���� ��1�֣� �� O=C=O ��2�֣�
2C+SiO2![]() 2CO+Si ��2�֣�
2CO+Si ��2�֣�
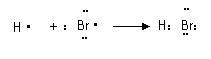 |
(2) �������� ���壨1�֣� ��2�֣�
2Fe2++2Br��+2Cl2=2Fe3++Br2+4Cl�� ��2�֣�
(3) Cu+2H2SO4��Ũ��![]() CuSO4+SO2��+2H2O��2�֣�
CuSO4+SO2��+2H2O��2�֣�
ȡ����C��Һ���Թ��У��μ�KSCN��Һ�����������ٵμ���ˮ��Ѫ��ɫ����2�֣�
0.56 ��2�֣�

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�