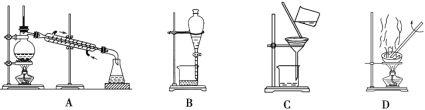
��Ŀ����
����Ŀ����(Ga)����(Ge)����(Si)����(Se)�ĵ��ʼ�ijЩ���������黯�ء����صȶ��dz��õİ뵼����ϡ��ش��������⣺
(1)��̬��ԭ�ӵĺ�������Ų�ʽΪ[Ar]__________���������ռ�ݵ�����ܲ�ķ�����________________��
(2)�����ࡢ����ͬ��������Ԫ�أ�������Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_______________________��
(3)��������н��ʯ�ṹ����ԭ��֮����ڵ�������Ϊ__________________��
(4)ˮ������Ҫ�ɷ��Ƕ������裬��ˮ���й�ԭ�ӵ���λ����____________�������������γ�һϵ�еĶ�Ԫ������SiH4��Si2H6�ȣ����ȡ��������γ�SiCl4��SiBr4�������������ʷе��ɸߵ���˳��Ϊ_______________������ϩ(Si4H8)��![]() ����
����![]() ������֮��Ϊ______________��
������֮��Ϊ______________��
(5)��֪GaCl3���۵�77. 9 ��C ���е�201��C �� GeCl4���۵� -49. 5 ��C ���е�84 ��C����GaCl3�ľ�������Ϊ______________��GaCl3���ӻ��������Ϊ_____________��GeCl4�Ŀռ乹��Ϊ_________________��
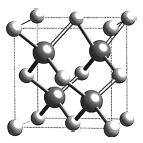
(6)�黯�ڵľ����ṹ��ͼ��ʾ�����þ����ܶ�Ϊ![]() ��������a =_____________cm (��NA��ʾ�����ӵ�����)��
��������a =_____________cm (��NA��ʾ�����ӵ�����)��
���𰸡�3d104s24p4 N As��Se��Ge ���ۼ� 4 SiBr4��SiCl4��Si2H6��SiH4 11��1 ���Ӿ��� sp2 �������� ![]()
��������
(1) Se��34��Ԫ�أ����ڵ������ڵڵڢ�A�壻���������Ӵ��ڵ��Ӳ���IJ㣻
(2) ͬ��������Ԫ����ԭ�����������һ�����ܳ��������ƣ���IIA�塢VA��Ԫ�ص�һ�����ܸ�������Ԫ�صģ�
(3) ��������н��ʯ�ͽṹ��Geԭ������Χ4��Geԭ���γ���������ṹ����ռ������������״�ṹ������ԭ�Ӿ��塣
(4) ˮ��������1����ԭ�ӽ��4����ԭ�ӣ�ͬʱÿ����ԭ�ӽ��2����ԭ�ӣ���[SiO4]������ṹ��ռ������������վ�ṹ�������ڷ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���е�Խ�ߣ�����ϩ(Si4H8)�붡ϩ�ṹ���ƣ���������8��Si-H����1��Si=Si˫����2��Si-Si��������Ϊ������˫������1��������1��������
(5)GaCl3���۷е�ܵͣ����Ϸ��Ӿ�������ʣ�GaCl3��Gaԭ���γ�3��Ga-Cl����û�йµ��Ӷԣ��ӻ������ĿΪ3��GeCl4��Geԭ���γ�4��Ge-Cl����û�йµ��Ӷԣ��۲���Ӷ���Ϊ4�����ռ乹����VSEPRģ����ͬ��
(6)��̯�����㾧����As��Gaԭ����Ŀ������ܶȼ��㾧�������������������η��ɵþ���������
(1) Se��34��Ԫ�أ����ڵ������ڵڢ�A�壬��������Ų�ʽΪ��[Ar]3d104s24p4 �����������Ӵ��ڵ��Ӳ���IJ㣬��������ռ�ݵ�����ܲ�ķ�����N��
(2) ͬ��������Ԫ����ԭ�����������һ�����ܳ��������ƣ���IIA�塢VA��Ԫ�ص�һ�����ܸ�������Ԫ�صģ��ʵ�һ������Ϊ��As��Se��Ge��
(3) ��������н��ʯ�ͽṹ��Geԭ������Χ4��Geԭ���γ���������ṹ����ռ������������״�ṹ������ԭ�Ӿ��壬Geԭ��֮���γɹ��ۼ���
(4) ˮ��������1����ԭ�ӽ��4����ԭ�ӣ�ͬʱÿ����ԭ�ӽ��2����ԭ�ӣ���[SiO4]������ṹ��ռ������������վ�ṹ��ˮ���ľ����й�ԭ�ӵ���λ��Ϊ4�������ڷ��Ӿ��壬��Է�������Խ���Ӽ�������Խǿ���е�Խ�ߣ��ʷе㣺SiBr4��SiCl4��Si2H6��SiH4������ϩ(Si4H8)�붡ϩ�ṹ���ƣ���������8��Si-H����1��Si=Si˫����2��Si-Si��������Ϊ������˫������1��������1����������������������������֮��Ϊ11��1��
(5)GaCl3���۷е�ܵͣ����ڷ��Ӿ��壻GaCl3��Gaԭ���γ�3��Ga-Cl����û�йµ��Ӷԣ��ӻ������ĿΪ3���ӻ���ʽΪsp2�ӻ���GeCl4��Geԭ���γ�4��Ge-Cl����û�йµ��Ӷԣ��۲���Ӷ���Ϊ4�����ռ乹����VSEPRģ����ͬ�����ռ乹��Ϊ�������壻
(6)�����к�ɫ����ĿΪ4����ɫ����ĿΪ8��![]() +6��
+6��![]() =4��������=4��
=4��������=4��![]() g���ʾ������=4��
g���ʾ������=4��![]() g�¦�gcm-3=
g�¦�gcm-3=![]() cm3���ʾ�������=
cm3���ʾ�������=![]() cm��
cm��

 �Ƹ������������ϵ�д�
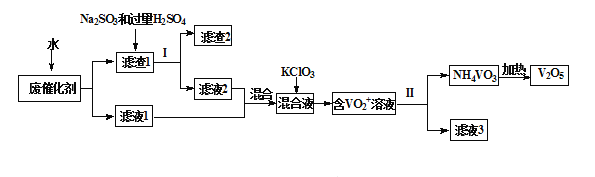
�Ƹ������������ϵ�д�����Ŀ�������������㷺����ұ�𡢻�������ҵ�������Ͻ����Ӽ������������ʯ�;����õĴ����ȡ�Ϊ�������ú���������������һ�����÷ϴ���������V2O5��VOSO4�������Թ����Σ�����V2O5���¹����������£�
��֪��a.���ֺ������ʳ�������ˮ�е��ܽ������±���ʾ��
���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
b.![]() +2OH-
+2OH-![]()
![]() +H2O
+H2O
�ش��������⣺
��1����ˮ���ݷϴ�����Ϊ����ߵ�λʱ���ڷϷ��Ľ����ʣ����Բ�ȡ�Ĵ�ʩΪ_________��дһ������
��2����Һ1����Һ2�з��Ĵ�����ʽ��ͬ���������ʽΪ_______________�������ӷ��ţ���
��3��������1�м���Na2SO3����H2SO4��Һ������Ӧ�Ļ�ѧ����ʽΪ_____________��
��4������VO2+�ķ�Ӧ������1molKClO3ʱת��6mol���ӣ��÷�Ӧ�����ӷ���ʽΪ________________
��5����ϻ�ѧ�����ƽ���ƶ�ԭ�����ͼ��백ˮ��һ������Ϊ__________________
��6�������NH4VO3����ʽ�����������Գ����ʣ�NH4VO3������V�������ͷϴ���V������֮�ȣ���ʾ�ò���Ӧ���Ļ����ʡ��������ͼ�¶ȳ���80���Ժ������½��Ŀ���ԭ����________________________��___________________��д��������
��7���ù��������п���ѭ�����õ�����Ϊ__________________��
��8���ⶨ��Ʒ��V2O5�Ĵ��ȣ���ȡa g��Ʒ�����������ܽ⣬�õ�(VO2)2SO4��Һ���ټ���b1 mL c1 mol��L1 (NH4)2Fe(SO4)2��Һ��VO2++2H++Fe2+==VO2++Fe3++H2O���������c2 mol��L1 KMnO4��Һ�ζ�������(NH4)2Fe(SO4)2���յ㣬����KMnO4��Һ�����Ϊb2 mL����֪����ԭΪMn2+���������ʲ����뷴Ӧ�����Ʒ��V2O5��Ħ��������182 g��mol1��������������______�����г�����ʽ��
����Ŀ���±��ж�Ӧ��ϵ��ȷ����
A | CH3CH3+Cl2 | ��Ϊȡ����Ӧ |
B | ����֬�õ����� | ��������ˮ�ⷴӦ |
C | Cl2+2Br=2Cl+Br2 | ��Ϊ���ʱ���ԭ���û���Ӧ |
D | 2Na2O2+2H2O+4NaOH+O2�� | ��Ϊˮ����ԭ����������ԭ��Ӧ |
����Ŀ����Դ����ϡ���Ϣһ�𱻳�Ϊ�ִ���ᷢչ������֧���������Դ�ݽߵ�Σ���������Դ�����ʺͿ�������Դ�ǽ����һ�����������Ҫ����
(1)��ѧ��Ӧ���ʺ���������������������أ����ǻ�ѧѧ�ƹ�ע�ķ���֮һ��ijѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯����400 mLϡ�����м���������п�ۣ�����ˮ���ռ���Ӧ�ų���������ʵ���¼����(�ۼ�ֵ)��
ʱ�� | 1 | 2 | 3 | 4 | 5 |
�������/mL(���) | 100 | 240 | 464 | 576 | 620 |
����һ��ʱ���ڷ�Ӧ�������______ min(����0��1����1��2����2��3����3��4������4��5��)��
����һѧ��Ϊ���Ʒ�Ӧ���ʷ�ֹ��Ӧ�������Բ�������������������������м���������������Һ�Լ�����Ӧ���ʵ���Ӱ��������������������Ϊ���е���______ (����ĸ���)��
A��KCl��Һ B��Ũ���� C������ˮ D��CuSO4��Һ
(2)��ͼΪԭ���װ��ʾ��ͼ��
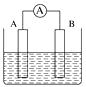
�ٽ���Ƭ��ͭƬ�õ���������һ�����Ũ�����У�һ������ռ���Һ�У��ֱ��γ���ԭ��أ���������ԭ����У��������ķֱ���_______������ĸ����
A����Ƭ��ͭƬ B��ͭƬ����Ƭ
C����Ƭ����Ƭ D��ͭƬ��ͭƬ
д������Ũ������Һ���γɵ�ԭ��ص�������Ӧʽ��_______��
����AΪCu��BΪʯī�������ΪFeCl3��Һ������ʱ���ܷ�ӦΪ2FeCl3+Cu=2FeCl2+CuCl2��д��B�缫��Ӧʽ��______���õ���ڹ���ʱ��A�缫��������______������������������С����������������
����Ŀ������ʵ�鷽������У����ﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ��ȥNaCl������������KNO3���� | ���̶�����ˮ�����Һ�������ᾧ�����ȹ��� |
B | ���������������Ƿ������� | ���뺬�з�̪��NaOH��Һ�����۲��²���Һ��ɫ�仯 |
C | ֤��Na2CO3��Һ�д���ˮ��ƽ�� | ���з�̪��Na2CO3��Һ�е���BaCl2��Һ���۲���Һ�ı仯 |
D | �Ƚ�Fe3+��I2��������ǿ�� | ���е��۵�KI��Һ�е���FeCl3��Һ���۲���Һ��ɫ�仯 |
A.AB.BC.CD.D