��Ŀ����
4������Cr3+���ӵĵ������������LED��ӫ��ۻ��ʲ��ϣ����������侧����ͼ1��ʾ�������Ȼ����백�����෴Ӧ�Ƶã�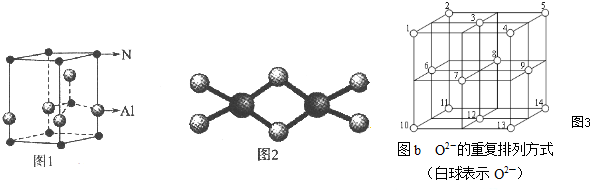
��1����̬Cr�ļ۵����Ų�ʽ�ɱ�ʾΪ3d54s1��
��2���������Ļ�ѧʽΪAlN��������ԭ������Ҿ�����ȵĵ�ԭ����ĿΪ4����
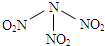
��3���Ȼ�������������˫����Al2Cl6�ṹ��ͼ2��ʾ����Al2Cl6�д��ڵĻ�ѧ���й��ۼ�����λ����
��4����ѧ��������Ƴ�������Ϊ��Ч����ƽ�����N��NO2��3����ͼ��ʾ����
��֪�÷�����N-N-N���Ƕ���108.1�㣬�����й�N��NO2��3��˵����ȷ����a��
a���������е�Ԫ�������ֲ�ͬ�Ļ��ϼ�
b�������ʿ��Թ������������������
c���÷����е�4����ԭ�ӹ�����������
d���÷����е�ԭ�ӵĻ�ѧ����������ͬ
��5��AlCl3��������Ӧ�������������Ӣ���̼ԭ�ӵ��ӻ�����Ϊsp3��sp2�ӻ���
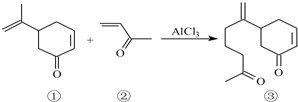
��6����ѧ���о�������Fe3O4����Fe2+��Fe3+��O2-ͨ�����Ӽ�����ɵĸ������Ӿ��壬O2-���ظ����з�ʽ��ͼ3��ʾ�������з�ʽ�д������������͵���O2-Χ�ɵĿ�϶����1��3��6��7��O2-Χ�ɵĿ�϶��3��6��7��8��9��12��O2-Χ�ɵĿ�϶��ǰ��Ϊ���������϶������Ϊ���������϶��Fe3O4����һ���Fe3+��������������϶�У���һ��Fe3+��Fe2+��������������϶�У���Fe3O4�����У����������϶����O2-������Ŀ֮��2��1��12.5%���������϶����Fe3+��
���� I����1��CrԪ��ԭ�Ӻ�����24�����ӣ����ݺ�������Ų�������д��۵����Ų�ʽ��
��2�����þ�̯�������֪������Al��Nԭ����Ŀ��Ϊ2��ȷ���仯ѧʽΪAlN���ɵ������ľ����ṹ����֪Nԭ����Χ��4��Alԭ�ӣ�����������Ϊ1��1�ͽṹ����Alԭ����Χ��4��Nԭ�ӣ�
��3���Ȼ�����������˵�����ڷ��Ӿ��壬Alԭ����Clԭ��֮���γɹ��ۼ�����������Alԭ����Clԭ��֮�仹�γ���λ����
��4��N��NO2��3��N-N-N���Ƕ���108.1�㣬�����ṹ��֪������Nԭ�ӻ����йµ��Ӷԣ�
a�������γɹ��ۼ���Ԫ�ؽ����жϣ�
b���û��������ڹ��ۻ����
c���������е�4����ԭ�ӹ����������壬������N-N-N���Ƕ���60�㣻
d���÷�����-NO2�еĵ�ԭ����ͬ��
��5�����Ӣ����о��γ�4��������̼ԭ�ӣ�̼��˫����̼̼˫����Cԭ�Ӿ��γ�3���Ҽ����ݴ��ж��ӻ���ʽ��
��6���ṹ����1��3��6��7��O2-Χ�ɵ����������϶��8�������ݾ�̯�����㾧��ṹ��O2-������Ŀ�������������������϶����O2-������Ŀ֮�ȣ���ϸ��ݻ��ϼ۴�����Ϊ0����ṹ�к���Fe3+��Fe2+��Ŀ�����ݡ�Fe3O4����һ���Fe3+��������������϶�У���һ��Fe3+��Fe2+��������������϶�С����ж����Fe3+�����������϶�е���Ŀ��������������������϶������ʣ�
��� I����1��CrԪ��ԭ�Ӻ�����24�����ӣ���������Ų�ʽΪ1s22s22p63s23p63d54s1����۵����Ų�ʽΪ��3d54s1��
�ʴ�Ϊ��3d54s1��
��2���ɾ����ṹ��֪��������Alԭ����ĿΪ1+4��$\frac{1}{4}$=2��Nԭ����ĿΪ1+8��$\frac{1}{8}$=2���ʵ�������ѧʽΪAlN���ɵ������ľ����ṹ����֪Nԭ����Χ��4��Alԭ�ӣ�����������Ϊ1��1�ͽṹ����Alԭ����Χ��4��Nԭ�ӣ�
�ʴ�Ϊ��AlN��4��
��3���Ȼ�����������˵�����ڷ��Ӿ��壬Alԭ����Clԭ��֮���γɹ��ۼ�����������Alԭ����Clԭ��֮�仹�γ���λ����
�ʴ�Ϊ�����ۼ�����λ����
��4��N��NO2��3��N-N-N���Ƕ���108.1�㣬�����ṹ��֪������Nԭ�ӻ����йµ��Ӷԣ�
a�����γɹ��ۼ���ԭ�ӿ�֪���÷�����NO2�ĵ�ԭ�ӺͶ����ϵ�Nԭ�Ӵ��ڵĻ�ѧ����ͬ�������仯�ϼ۲�ͬ����a��ȷ��
b�������ʵĹ������Ƿ��ӣ����ڹ��ۻ�������������εĹ��������������ӣ��������ӻ�������Ը����ʲ��ܹ�������������������b����
c���������е�4����ԭ�ӹ����������壬������N-N-N���Ƕ���60�㣬��������N-N-N���Ƕ���108.1�㣬ӦΪ�����Σ���c����
d������Nԭ�����ӵ�ԭ�ӻ���ſ�֪���÷�����NO2�еĵ�ԭ�ӻ�ѧ������ͬ����d����
��ѡ��a��
��5�����Ӣ����о��γ�4��������̼ԭ�ӣ�̼��˫����̼̼˫����Cԭ�Ӿ��γ�3���Ҽ���̼ԭ�Ӿ�û�йµ��Ӷԣ��ӻ������ĿΪ4��3����̼ԭ�Ӳ�ȡsp3��sp2�ӻ���
�ʴ�Ϊ��sp3��sp2�ӻ���
��6���ṹ����1��3��6��7��O2-Χ�ɵ����������϶��8������ͼ��֪����ṹ��O2-������ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�������������϶����O2-������Ŀ֮��8��4=2��1��
���ݻ��ϼ۴�����Ϊ0����ṹ�к���2��Fe3+��1��Fe2+��Fe3O4����һ���Fe3+��������������϶�У���һ��Fe3+��Fe2+��������������϶�У�����������������϶�е�Fe3+Ϊ1������������������϶����1��Fe3+��1��Fe2+������Fe3+�����������϶�������������϶��$\frac{1}{8}$��100%=12.5%��
�ʴ�Ϊ��2��1��12.5��
���� ���⿼�龧�����㡢���ӽṹ����������Ų�����ѧ�����ӻ�����ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�ؼ��ǽ���Ľṹ�����⣬�Ѷ��еȣ�

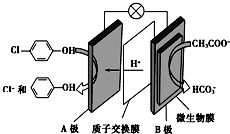 �������ƺͶ��ȷӣ�
�������ƺͶ��ȷӣ�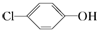 ���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ�������й�˵������ȷ���ǣ�������
���ķ�ˮ�������������س�ȥ����ԭ����ͼ��ʾ�������й�˵������ȷ���ǣ�������| A�� | �缫B�Ǹ��� | |
| B�� | ���Ӵ�A������B�� | |
| C�� | B���ĵ缫��Ӧʽ��CH3COO--8e-+2H2O=2CO2+7H+ | |
| D�� | ������ķ�ˮpH���� |
| A�� | ŷ�ͱ�����һЩ������Ͽն�������Ũ�Ⱥܸߣ���ԭ������Щ�������Ṥҵ���� | |
| B�� | ������Ⱦ���еĶ���������������������� | |
| C�� | ��Ȼ��ҩ�����κζ������ã��ɳ��ڷ��� | |
| D�� | ����ʳ�ö��������������ʳƷ���ƻ�ά����B1����ͨ������������ʣ���NaOH���ķ������������������ж� |
| A�� | ˮ���ӵı���ģ�ͣ� | B�� | F-�Ľṹʾ��ͼ�� | ||
| C�� | H2O2�ĵ���ʽ��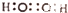 | D�� | �����ǵĽṹ��ʽ��C6H12O6 |
| A�� | ���ռ���Һ�м�������Al+2OH-=AlO2-+H2�� | |
| B�� | �ù�����ˮ�����̵����е�SO2��SO2+2NH3•H2O=SO32-+2NH4++H2O | |
| C�� | ������FeCl3��Һ�����ˮ����ȡFe��OH��3���壺Fe3++3H2O?Fe��OH��3��+3H+ | |
| D�� | ��Fe2+������ˮ������ClO2-��ԭ��ΪCl-��4Fe2++ClO2-+4H+=4Fe3++Cl-+2H2O |
| A�� | ȼú�м���CaO��ɼ�������������ŷ��� | |
| B�� | ���ࡢ��֬�������ʶ���������Ҫ��Ӫ�����ʣ���������Ȼ�߷��ӻ����� | |
| C�� | Ư�ۡ�����������������ˮ�������仯ѧԭ����ͬ | |
| D�� | ��ȫ��ÿ�걻��ʴ�Ľ����У��绯ѧ��ʴ�Ȼ�ѧ��ʴ��ռ������ |
| ѡ�� | ʵ��Ŀ�� | ʵ����� |
| A | ����Fe2��SO4��3��Һ���Ƿ���FeSO4 | ȡ������Һ���Թܣ����뼸��0.1mol/L KMnO4��Һ |
| B | ֤��Al��OH��3�������������� | ȡAl��OH��3���Թ�A��B���ֱ�μӰ�ˮ������ |
| C | ʹ�������ܱ��� | ������Һ�У����뱥�ͣ�NH4��2SO4��Һ���������� |
| D | ��ȥNa2CO3�е�NaCl | �ܽ⣬����AgNO3��Һ�����ٲ������������ã����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��
��