��Ŀ����
����Ŀ��A��B��C��D�� E��F��G��H��Ԫ�����ڱ�ǰ�����ڳ���Ԫ�أ���ԭ���������������������Ϣ���±���
Ԫ�� | �����Ϣ |
A | ԭ�Ӻ�����6�ֲ�ͬ�˶�״̬�ĵ��� |
C | ��̬ԭ����s����������p����������� |
D | ԭ�Ӱ뾶��ͬ����Ԫ������� |
E | ��̬ԭ�����������Ų�ʽΪ3s23p1 |
F | ��̬ԭ�ӵ������p������������ӵ������������������ӵ����������෴ |
G | ��̬ԭ�Ӻ�����7���ܼ���������ߵ��ܼ�����6������ |
H | ���ҹ�ʹ������ĺϽ��е�����ҪԪ�� |
���û�ѧ������գ�
(1)AԪ��λ��Ԫ�����ڱ���_______����_______�壻BԪ�غ�CԪ�صĵ�һ�����ܱȽϣ��ϴ����________��CԪ�غ�FԪ�صĵ縺�ԱȽϣ���С����________��
(2)BԪ���������к�����ḻ��Ԫ���γɵ��������ķ���ģ��Ϊ________��BԪ�����γɵĵ��ʷ���![]() ����������Ŀ֮��Ϊ________��
����������Ŀ֮��Ϊ________��
(3)GԪ�صĵͼ������ӵ����ӽṹʾ��ͼ��________��FԪ��ԭ�ӵļ۵��ӵĹ����ʾʽ��________��HԪ�صĻ�̬ԭ�Ӻ�������Ų�ʽ��________��
(4)G�ĸ������ӵ���Һ��H���ʷ�Ӧ�����ӷ���ʽΪ_________________����EԪ�سɶԽ��߹�ϵ��ijԪ�ص�����������ˮ����������ԣ�д��������������DԪ�ص�����������ˮ���ﷴӦ�����ӷ���ʽ��_________________��
���𰸡��� ��A N Cl ���� 1��2 ![]()
![]() 1s22s22p63s23p63d104s1��[Ar]3d104s1 2Fe3++3Cu=2Fe2++Cu2+ Be(OH)2+2NaOH=Na2BeO2+2H2O
1s22s22p63s23p63d104s1��[Ar]3d104s1 2Fe3++3Cu=2Fe2++Cu2+ Be(OH)2+2NaOH=Na2BeO2+2H2O
��������
A��B��C��D��E��F��G��H��Ԫ�����ڱ�ǰ�����ڳ���Ԫ�أ���ԭ��������������Aԭ�Ӻ�����6�ֲ�ͬ�˶�״̬�ĵ��ӣ���AΪ̼Ԫ�أ�E��̬ԭ�����������Ų�ʽΪ3s23p1����EΪAlԪ�أ�Dԭ�Ӱ뾶��ͬ����Ԫ���������ԭ������С��Al������̼���ʴ��ڵ���������A�壬��DΪNaԪ�أ�C��̬ԭ����s����������p����������ȣ�ԭ������С��Na��ԭ�Ӻ�������Ų�Ϊ1s22s22p4����CΪOԪ�أ�B��ԭ����������̼����֮�䣬��BΪNԪ�أ�G��̬ԭ�Ӻ�����7���ܼ���������ߵ��ܼ�����6�����ӣ���������Ų�ʽΪ1s22s22p63s23p63d64s2����GΪFeԪ�أ�F��̬ԭ�ӵ������p������������ӵ������������������ӵ����������෴����Χ�����Ų�Ϊns2np5�����ԭ��������֪��FΪClԪ�أ�H���ҹ�ʹ������ĺϽ��е�����ҪԪ�أ���HΪCuԪ�أ��ݴ˷������
��������������AΪ̼Ԫ�أ�BΪNԪ�أ�CΪOԪ�أ�DΪNaԪ�أ�EΪAlԪ�أ�FΪClԪ�أ�GΪFeԪ�أ�HΪCuԪ�ء�
(1)AΪ̼Ԫ�أ�λ��Ԫ�����ڱ��ڶ�������A�壬NԪ��ԭ��2p���Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���OԪ�أ�O��Cl�γɵĻ�������OԪ�ر��ָ��ۣ��Լ��ϵ��ӵ�����������ǿ����Cl�ĵ縺�Խ�С���ʴ�Ϊ��������A��N��Cl��
(2)NԪ���������к�����ḻ��Ԫ���γɵ��������ΪNH3�����ӹ���Ϊ�����Σ�NԪ�����γɵĵ��ʷ��ӽṹʽΪN��N������������������Ŀ֮��Ϊ1��2���ʴ�Ϊ�������Σ�1��2��
(3)GΪFeԪ�أ���ͼ������ӵ����ӽṹʾ��ͼ�� ��FΪClԪ�أ���ԭ�ӵļ۵��ӹ����ʾʽΪ
��FΪClԪ�أ���ԭ�ӵļ۵��ӹ����ʾʽΪ![]() ��HΪCuԪ�أ����̬ԭ�Ӻ�������Ų�ʽ��1s22s22p63s23p63d104s1���ʴ�Ϊ��
��HΪCuԪ�أ����̬ԭ�Ӻ�������Ų�ʽ��1s22s22p63s23p63d104s1���ʴ�Ϊ�� ��
��![]() ��1s22s22p63s23p63d104s1��
��1s22s22p63s23p63d104s1��
(4)��������Cu��Ӧ��������������ͭ���ӣ���Ӧ�����ӷ���ʽΪ��2Fe3++Cu=2Fe2++Cu2+����E(Al)Ԫ�سɶԽǹ�ϵ��ijԪ�ص�����������ˮ����������ԣ���Ԫ��ΪBe�������������ΪBe(OH)2�����������Ʒ�Ӧ����ʽΪ��Be(OH)2+2NaOH=Na2BeO2+2H2O���ʴ�Ϊ��2Fe3++Cu=2Fe2++Cu2+��Be(OH)2+2NaOH=Na2BeO2+2H2O��

����Ŀ����þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO��ռ40%������������������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ��Դ�Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO47H2O�Ĺ����������£�
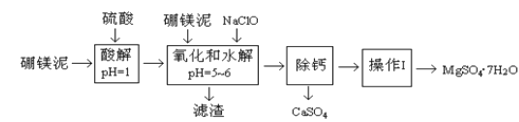
��1��ʵ������Ҫ1 mol/L������980 mL������ 98% ��Ũ���ᣨ��= 1.84 g/mL�������ƣ���ȡŨ������Ҫʹ����Ͳ�Ĺ��Ϊ____����дѡ����ĸ����
A��10 mL B��20 mL C��50 mL D��100 mL
��2�������NaClO����Mn2+ ��Ӧ��Mn2+ + ClO + H2O = MnO2��+ 2H+ + Cl ���ڸò����л���һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ________��
��3����������Ҫ�ɷֳ�����Fe(OH)3��Al(OH)3�⣬������______��____��
��4���ڡ����ơ�ǰ���������Һ��Fe3+ �Ƿ������������鷽��_____����д������������ͽ��ۣ�
��5����֪MgSO4��CaSO4 ���ܽ�ȣ���λΪ g/100 g ˮ�����±���
�¶ȣ��棩 | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
�����ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵����������_______�����������ǽ���Һ��������Ũ������ȴ�ᾧ��______����õ���MgSO47H2O��
��6��ʵ�����ṩ����þ�100 g ���õ� MgSO47H2O Ϊ196.8 g ����MgSO47H2O �IJ���Ϊ____��
����Ŀ��(һ)Na��Cu��O��Si��S��Cl�dz���������Ԫ�أ�
(1)Naλ��Ԫ�����ڱ���__���ڵ�__�壻S�Ļ�̬ԭ�Ӻ�����__��δ�ɶԵ��ӣ�
Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ__��
(2)������������������գ�
��һ������ | ���Ӱ뾶 | �۵� | ���� |
Si______S | O2-______Na+ | NaCl ______ Si | H2SO4 __________HClO4 |
(3)ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡ��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ___
(��).ijԪ�ص�ԭ������Ϊ33����ش�
(1)��Ԫ��ԭ�Ӻ�����_______�����Ӳ㣬______���ܼ���______��ԭ�ӹ����
(2)�������������Ų�ʽΪ____________�����ĵ����Ų�ʽΪ________�������ʾʽΪ_______________��