��Ŀ����
15��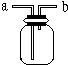 ��ͼ��ʾ����ѧ��ѧʵ���г�����װ�ã����ж�����;��
��ͼ��ʾ����ѧ��ѧʵ���г�����װ�ã����ж�����;����1����ƿ��װ��X��Һ����CO��CO2�Ļ��������a�ܿ�ͨ�룬���Գ�ȥCO2����XΪB����ѡ�ţ�����д����Ӧ�Ļ�ѧ����ʽCO2+2NaOH=Na2CO3+H2O�������ȥX 20g������������CO2�����Ϊ5.6L��
A��H2SO4 B��NaOH C��NaCl D��HCl
��2�������ſ������ռ�H2����H2����Ӧ��b�����ţ���ͬ���ܿڵ��룻�����ſ������ռ�CO2����CO2����Ӧ��a �ܿڵ��룮
��3��ҽԺ�����������ʱ��������������ƿ�벡�˺������֮�䰲װ��ˮ�ĸ�װ�ã��۲����ݲ�����������Ա���ڹ������ʣ���ʱ����Ӧ��a�ܿڵ��룮
���� ��1��CO2��Ӧ����CO���ܣ����CO2+2NaOH=Na2CO3+H2O���㣻
��2���������ܶȱȿ���С�����������ſ������ռ�����������̼���ܶȱȿ����ܶȴ����������ſ������ռ���
��3������������ˮ����װ��ˮ�ĸ�װ�ã��۲����ݴ��µ����ݳ��������Ա���ڹ������ʣ�
��� �⣺��1��CO2��Ӧ����CO���ܣ����߾��������ᡢNaCl��HCl��Ӧ����XΪNaOH������CO2+2NaOH=Na2CO3+H2O����ȥX 20g��n��NaOH��=$\frac{20g}{40g/mol}$=0.5mol������������CO2�����Ϊ0.5mol��$\frac{1}{2}$��22.4L/mol=5.6L��
�ʴ�Ϊ��B��CO2+2NaOH=Na2CO3+H2O��5.6L��
��2���������ܶȱȿ���С�����������ſ������ռ�����H2����Ӧ��b�ܿڵ��룻��������̼���ܶȱȿ����ܶȴ����������ſ������ռ�����CO2����Ӧ��a �ܿڵ��룬
�ʴ�Ϊ��b��a��
��3������������ˮ����װ��ˮ�ĸ�װ�ã��۲����ݴ��µ����ݳ��������Ա���ڹ������ʣ����ʱ����Ӧ��a�ܿڵ��룬����b�����ɽ�ˮ�ų����ʴ�Ϊ��a��
���� ���⿼��ʵ��װ�õ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬������������ʡ����������ᴿ��������ռ�������Ϊ���Ĺؼ���ע�ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | 1mol Na�μ�������ԭ��Ӧʱ����ת��NA | |
| B�� | 3.2g SO2��2.3g NO2�����е���ԭ������� | |
| C�� | ��״���£�2.24L SO3���е�Oԭ����Ϊ0.3NA | |
| D�� | 1L 0.1mol/LMgCl2��Һ��Cl-������Ϊ0.2NA |
| A�� | 1000mL0.1mol/L ��NaCl��Һ�У�Na+��Cl-��������Ϊ0.2NA | |
| B�� | 2NA��������̼���ӵ�����Ϊ44g | |
| C�� | NA������������ռ�����Ϊ22.4L | |
| D�� | 17g����������ԭ����ΪNA |
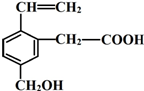 ij�л���Ľṹ��ʽ��ͼ�������ʿ����еĻ�ѧ�����ǣ�������
ij�л���Ľṹ��ʽ��ͼ�������ʿ����еĻ�ѧ�����ǣ��������ٿ�ȼ�գ�
�ڿɸ���ӳɣ�
�ۿ�ʹ����KMnO4��Һ��ɫ��
�ܿɸ�NaHCO3��Һ��Ӧ��
�ݿɸ�NaOH��Һ��Ӧ��
��1mol���л���������Na��Ӧ����1mol H2��
| A�� | �٢ڢۢ� | B�� | �٢ڢܢ� | C�� | �٢ڢۢܢ� | D�� | ȫ�� |
| ѡ�� | ���� | ���� | ���� |
| A | �����½�CuƬ����ŨH2SO4�� | ���ɴ̼�����ζ���� | Cu��ŨH2SO4��Ӧ����SO2 |
| B | Al2��SO4��3��Һ�еμӹ�����ˮ | ���ɰ�ɫ��״���� | Al��OH��3�����ڰ�ˮ |
| C | ��ij��Һ�м���������ˮ��������Һ�м���KSCN��Һ | ��Һ���ɫ | ����Һ�к���Fe2+ |
| D | ��ij��Һ�м���CCl4������ | Һ��ֲ㣬�²���Ϻ�ɫ | ����Һ�д���I- |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �ﵽ��ѧƽ��ʱ��4v����O2��=5v�棨NO�� | |
| B�� | ����λʱ��������x mol NO��ͬʱ������x molNH3����Ӧ�ﵽƽ�� | |
| C�� | �ﵽ��ѧƽ��ʱ��������������������� ��Ӧ���ʼ��٣��淴Ӧ�������� | |
| D�� | ��ѧ��Ӧ���ʹ�ϵ��2v����NH3��=3v����H2O�� |

| A�� | �������Ϊ��ˮ����ͨ����������з��룬�����Լ�ΪCCl4Һ�壬�����Һ��ֲ㣬�ϲ�ΪX��CCl4��Һ | |
| B�� | ��Ϊ���ͱ��ӵĻ�����ͨ����������з��룬�����Լ�ΪŨ��ˮ��XΪ�� | |
| C�� | ��Ϊ�屽����Ļ�����ͨ����������з��룬�����Լ�ΪNaOH��Һ��XΪ�屽 | |
| D�� | ��Ϊ����ͼ�Ȳ�Ļ�����ͨ����������з��룬�����Լ�Ϊ��ˮ��XΪ���� |
| A�� | һ���ǹ��ۼ� | B�� | һ�������Ӽ� | ||
| C�� | �����ǹ��ۼ���Ҳ���������Ӽ� | D�� | ����˵��������ȷ |