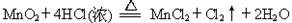��Ŀ����
�й�����ֵ�������ʾ��2013��ȫ��ƽ����������Ϊ52����֮��γ���������Ҫ�ɷ�Ϊ�������������ŷŵķ���������β�����ﳾ�ȡ�
��1����CH4������������β���е����������Ⱦ��
��֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(l) ��H����955 kJ/mol
2NO2(g)��N2O4(g) ��H����56.9 kJ/mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ ��
��2����֪��CO(g)��H2O(g) CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g)
CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g) CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ��
CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ��
��3����ʽ��������������������Ҫ����������
����Al2(SO4)3��Һ��Ͷ���ĩ״ʯ��ʯ�����ɼ�ʽ������[Al2(SO4)3��Al2O3]��Һ��
�ڼ�ʽ����������SO2��Al2(SO4)3��Al2O3+3SO2��Al2(SO4)3��Al2(SO3)3����д��Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ļ�ѧ����ʽ ��
�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3����ѡ��������Ϊ ������ţ�
| A��Ũ���� | B��KMnO4��Һ | C��5%��H2O2��Һ | D������ |
��1��CH4(g)+N2O4(g) = N2(g) +2H2O(l) + CO2(g) ��H=" ��898.1" kJ/mol
��2��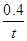 mol/( L��min) 2.25 24.6
mol/( L��min) 2.25 24.6
��3��Al2(SO4)3��Al2O3+3H2O+10NaOH=4Na[Al(OH)4]+3Na2SO4��C D������Al2(SO4)3ѭ��ʹ�á�
�������������(1) ����ʽH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(l) ��H����955 kJ/mol ��ȥ��ʽ2NO2(g)��N2O4(g) ��H����56.9 kJ/mol.�����ɵã�CH4(g)+N2O4(g) = N2(g) +2H2O(l) + CO2(g) ��H=" ��898.1" kJ/mol ����2���ɷ���ʽ���Կ�����ÿ����1mol��H2���ų�����41kJ�����ڷų�����32.8kJ�������H2�����ʵ���Ϊ32.8��41=0.8mol�������tmin��H2��ʾ��ƽ����Ӧ����Ϊv(H2)=��c/��t=0.8mol��2L��tmin=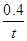 mol/( L��min). (2) ���ڷ�ӦCO(g)��H2O(g)
mol/( L��min). (2) ���ڷ�ӦCO(g)��H2O(g)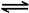 CO2(g)��H2(g)��˵�� ����Ӧ�ﵽƽ��ʱ��c(CO)=c(H2O)=(2.0-0.8)mol��2L=0.6mol/L ;c(CO2)=c(H2)=0.8mol��2L=0.4mol/L;�÷�Ӧ�Ļ�ѧƽ�ⳣ��
CO2(g)��H2(g)��˵�� ����Ӧ�ﵽƽ��ʱ��c(CO)=c(H2O)=(2.0-0.8)mol��2L=0.6mol/L ;c(CO2)=c(H2)=0.8mol��2L=0.4mol/L;�÷�Ӧ�Ļ�ѧƽ�ⳣ��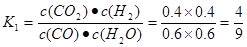 ����Ӧ CO2(g)��H2(g)
����Ӧ CO2(g)��H2(g)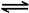 CO(g)��H2O(g)��CO(g)��H2O(g)
CO(g)��H2O(g)��CO(g)��H2O(g)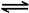 CO2(g)��H2(g)���淴Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ
CO2(g)��H2(g)���淴Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ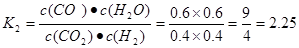 .����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2�����練Ӧ�ﵽƽ��ʱ������COΪxmol,��ˮ����Ҳ��xmol,δ��Ӧ��CO2(g)��H2(g)�����ʵ�������(1.0-x)mol.
.����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2�����練Ӧ�ﵽƽ��ʱ������COΪxmol,��ˮ����Ҳ��xmol,δ��Ӧ��CO2(g)��H2(g)�����ʵ�������(1.0-x)mol.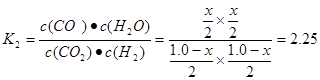 .���x=0.6mol.��˷�Ӧ�ﵽƽ������յ�����Ϊ0.6mol��41kJ/mol=24.6kJ.(3)��ΪAl2(SO4)3��Al2O3������NaOH������Ӧ�����Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ϳ��Կ�����Al2(SO4)3��Al2O3�Ļ������NaOH��Һ�����ķ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ:Al2(SO4)3��Al2O3+3H2O+10NaOH=4Na[Al(OH)4]+3Na2SO4.�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3ʱѡ������������������µ��������ӣ���������������������׳�ȥ��������Ŀ�ṩ���Լ���ѡ����ɫ������5%��H2O2��Һ����������ѡ��ΪC D���ò���Ӧ��Ŀ���ǽ�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3���Դﵽ���ʵ�ѭ�����ã���߾���Ч�档
.���x=0.6mol.��˷�Ӧ�ﵽƽ������յ�����Ϊ0.6mol��41kJ/mol=24.6kJ.(3)��ΪAl2(SO4)3��Al2O3������NaOH������Ӧ�����Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ϳ��Կ�����Al2(SO4)3��Al2O3�Ļ������NaOH��Һ�����ķ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ:Al2(SO4)3��Al2O3+3H2O+10NaOH=4Na[Al(OH)4]+3Na2SO4.�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3ʱѡ������������������µ��������ӣ���������������������׳�ȥ��������Ŀ�ṩ���Լ���ѡ����ɫ������5%��H2O2��Һ����������ѡ��ΪC D���ò���Ӧ��Ŀ���ǽ�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3���Դﵽ���ʵ�ѭ�����ã���߾���Ч�档
���㣺�����Ȼ�ѧ����ʽ����д����ѧ��Ӧ���ʡ���ѧƽ������ļ��㡢���淴Ӧ����ЧӦ����ʽ����������SO2�ķ�Ӧԭ����֪ʶ��

 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�Ϊ̽����ҵ����������ͭ�Ͻ���ϵ�������,��ͬѧ��Ƶ�ʵ�鷽������: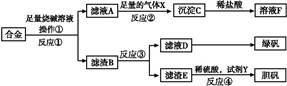
��ش�:
(1)�������õ��IJ��������� ��
(2)д����Ӧ�ٵĻ�ѧ����ʽ: ,��Ӧ�ڵ����ӷ�Ӧ����ʽ: ��
(3)���ʵ�鷽��,�����ҺD�к��еĽ�������(�Լ���ѡ) ��
(4)������E�м���ϡ������Լ�Y�Ƶ���������һ����ɫ��ѧ����,�Լ�YΪ��ɫҺ��,��Ӧ�ܵ��ܻ�ѧ����ʽ�� ��
(5)��ͬѧ�ڼ�ͬѧ�����Ļ��������������B���Ʊ�FeCl3��6H2O����,�������еμ�����ʱ,���ַ�Ӧ���ʱ�ͬŨ�������봿���۷�ӦҪ��,��ԭ���� ��
�������Ȼ�����Һ�ü���Ũ�������½ᾧ���Ƶ�FeCl3��6H2O ����,������ֱ�������ᾧ�ķ������Ƶþ���������� ��
(6)������B�ľ��Ȼ����ƽ���ֳ��ĵȷ�,�ֱ����ͬŨ�ȵ�ϡ����,��ַ�Ӧ��,�ڱ�״��������NO�������ʣ��������������±�(������Ļ�ԭ����ֻ��NO)��
| ʵ���� | �� | �� | �� | �� |
| ϡ�������(mL) | 100 | 200 | 300 | 400 |
| ʣ���������(g) | 9.0 | 4.8 | 0 | 0 |
| NO���(L) | 1.12 | 2.24 | 3.36 | V |
�������Ũ��Ϊ ;�����ܽ�ͭ������Ϊ ;����V= ��
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�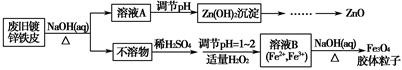
��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
��3������ҺB�Ƶ�Fe3O4�������ӵĹ����У������ͨ��N2����ԭ����_________________________________________________________��
��4�����ظ���ط���һ��������ԭ�ζ������ɲⶨ����Fe3O4�еĶ�������������������Ũ��Ϊ0.010 00 mol��L��1��K2Cr2O7����Һ250 mL��Ӧȷ��ȡ________g K2Cr2O7������4λ��Ч���֣���֪MK2Cr2O7��294.0 g��mol��1�������Ƹñ���Һʱ�����������в���Ҫ�õ�����________���ñ�ű�ʾ����
�ٵ�����ƽ�����ձ�������Ͳ���ܲ�������������ƿ����ͷ�ιܣ�����Һ��
��5���ζ������У�����ζ�ǰװ��K2Cr2O7����Һ�ĵζ��ܼ��첿�������ݣ����ζ�������������ʧ����ⶨ�����________���ƫ��ƫС�����䡱����
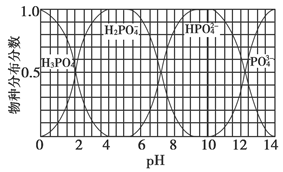
 ���뼾���Ĵ���
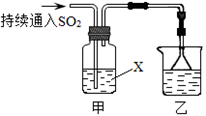
���뼾���Ĵ��� �������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
�������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
 Ca2��+
Ca2��+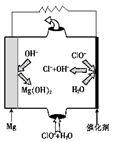
 ��
�� 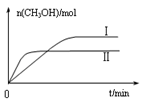
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H