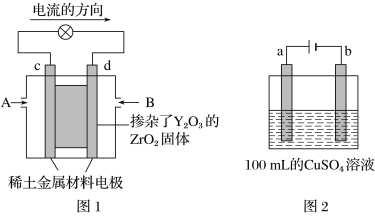
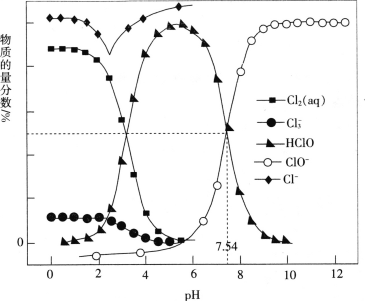
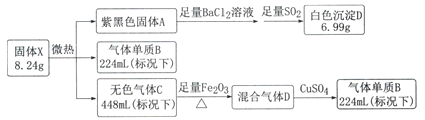
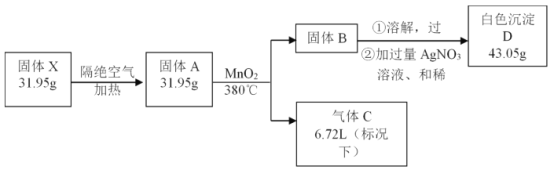
��Ŀ����
����Ŀ�������ĩX�п��ܺ���Fe��Fe2O3��CuO��Na2CO3��NH4Cl��K2SO4�е������֡�Ϊȷ���ù����ĩ�ijɷ֣���������ʵ�飺
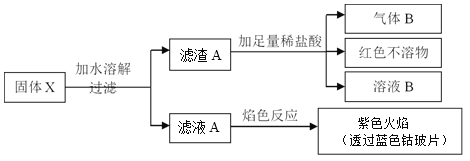
�����й�˵����ȷ����
A.��ҺA��һ������K2SO4������Na2CO3
B.����A��һ������Fe��CuO
C.�ж���ҺB���Ƿ���Fe3������ȡ��ҺB�������Թܣ��μ�KSCN��Һ������Һ��Ѫ��ɫ��˵����ҺB�к���Fe3��
D.ȡ��ҺA�������Թܣ��μ�0.1 mol��L-1NaOH��Һ���Թܿ�ʪ��ĺ�ɫʯ����ֽ��������˵������NH4Cl
���𰸡�B
��������
A��ȡ������ҺA������ɫ��Ӧ��������ɫ�ܲ����۲쵽�������ɫ��˵����Һ�к���K+������ҺA��һ������K2SO4��������ȷ���Ƿ���Na+����˲���ȷ���Ƿ���Na2CO3��A����
B��������A�м�������ϡ�����������B��˵��X�к���Fe�������˷�Ӧ��Fe+2HCl=FeCl2+H2����ͬʱ�к�ɫ������ú�ɫ������������Cu���ʣ�˵������A���е�CuO�������˷�Ӧ��CuO+2HCl=CuCl2+H2O��Fe+CuCl2=FeCl2+Cu��˵��X�к���CuO��B��ȷ��
C������B���������ɫ������ΪCu�����ݷ�Ӧ��Cu+2Fe3+=Cu2++2Fe2+����֪����ҺB��һ������Fe3+���������Fe3+��C����
D��ȡ��ҺA�������Թܣ��μ�0.1 mol��L-1NaOH��Һ���Թܿ�ʪ��ĺ�ɫʯ����ֽ��������������ԭ����X�в���NH4Cl��Ҳ��������ҺŨ�ȹ�С�����е�NH4+��OH-����γ�NH3��H2O��NH3��H2Oû�зֽ����NH3����˲��ܾݴ�ȷ������X���Ƿ�NH4Cl��D����
�ʴ�ѡB��