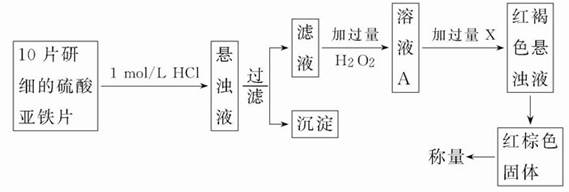
��Ŀ����
�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO��Cr2O3������ΪSiO2��Al2O3��Ϊԭ�ϲ�����ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ��������ͼ���漰����Ҫ��Ӧ��6FeO��Cr2O3+24NaOH+7KClO3 12Na2CrO4+3Fe2O3+7KCl+12H2O��
12Na2CrO4+3Fe2O3+7KCl+12H2O��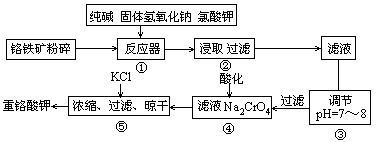
��1�����ǰ������������������
��2������۵���pH����˵õ��������� ��
��3���������У��ữʱ��CrO2- 4ת��ΪCr2O2- 7��д��ƽ��ת�������ӷ���ʽ
��
��4���ü�Ҫ������˵�������ݼ���KCl��ԭ�� ��
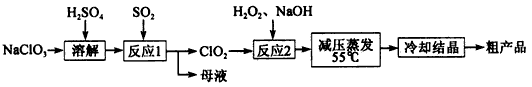
��5����ȡ�ظ��������2.500g���250mL��Һ��ȡ��25mL�����ƿ�У�����10mL2mol/ LH2SO4�������⻯�أ����Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���I2+2S2O2- 3��2I- +S4O2- 6��
���жϴﵽ�ζ��յ�������� ��
����ʵ���й���ȥNa2S2O3����Һ40.00mL�������ò�Ʒ���ظ���صĴ���Ϊ���������������������ʲ��μӷ�Ӧ�� ������2λ��Ч���֣���
(14��)
��1������Ӵ����������Ӧ���ʣ�2�֣�
��2��Al(OH)3��H2SiO3��2�֣�
��3��2 CrO2- 4+2H+ Cr2O2- 7+H2O��2�֣�
Cr2O2- 7+H2O��2�֣�
��4���¶ȶ��Ȼ��Ƶ��ܽ��Ӱ��С�������ظ���ص��ܽ��Ӱ��ϴ����ø��ֽⷴӦ���ɵõ��ظ���أ�2�֣�
��5���ٵ��μ����һ�������������Һ����Һ��ɫ��ɫ��2�֣���94.08 %��2�֣�
�������������
��1������������������������Ӵ����������Ӧ���ʣ�
��2�����ݹ�������ͼ�п��Կ����ڷ�Ӧ����SiO2��Al2O3��NaOH��Ӧ�ֱ�ת��ΪNa2SiO3��NaAlO2, ����۵���pH����˵õ���������Al(OH)3��H2SiO3��
��3���ữʱ��CrO2- 4ת��ΪCr2O2- 7ƽ��ת�������ӷ���ʽΪ2CrO2- 4+2H+ Cr2O2- 7+H2O��
Cr2O2- 7+H2O��
��4�����������Ϣ�¶ȶ��Ȼ��Ƶ��ܽ��Ӱ��С�������ظ���ص��ܽ��Ӱ��ϴ����ø��ֽⷴӦ�����½ᾧʱ�õ������ظ���ء�
��5�����ⵥ�ʴ��ڿ��õ��ۣ�ֱ����ɫ��ȥ�������ɵù�ϵʽ��Cr2O2- 7~3I2~6S2O2- 3��n(S2O2- 3)=0.04L��0.12mol/L����25ml��1/6n(S2O2- 3)= n(Cr2O2- 7)=0.0008mol����250ml�к��е�m(K2Cr2O7)=0.0008mol��10��294g/mol=2.352g����֪��������Ϊ2.352g/2.500g��100%=94.08%��
���㣺�����Թ�������Ϊ����������Ԫ�ؼ��������ѧʵ�������������ѧ��������֪ʶ��

������ʵ����ص������У���ȷ���� �� ��
| A������ˮ�����Һ©�����ټ�����������������á����ˣ��ɴӵ�ˮ�л�ȡ�� |
| B����CuSO4��Һ�õ�CuSO4��5H2O�IJ����Ǽ���Ũ������ȴ�ᾧ �����ˡ�ϴ�ӡ����� |
| C������������з�̪��NaOH��Һ�еμ�ϡ���ᵱ��ɫǡ����ȥʱ������ҺpH=7 |
| D����ij��Һ�еμ���ˮ�μ�KSCN��Һ����Һ��죬֤����Һ�к���Fe2+ |
(15��)��ʽ̼��ͭ�ijɷ��ж��֣��仯ѧʽһ��ɱ�ʾΪxCu(OH)2��yCuCO3��
(1)��ȸʯ����ɫ����һ������ı�ʯ������Ҫ�ɷ���Cu(OH)2��CuCO3��ij��ȤС��Ϊ̽����ȡ��ȸʯ����ѷ�Ӧ���������������ʵ�飺
ʵ��1����2.0mL 0.50 mol��L�C1��Cu(NO3)2��Һ��2.0mL 0.50 mol��L�C1��NaOH��Һ��0.25 mol��L�C1��Na2CO3��Һ��������ʾ�����ϡ�
ʵ��2�������ʱ����Ļ�����ڱ�����ʾ�¶��·�Ӧ��
ʵ���¼���£�
���� ����
| ��� | V (Na2CO3)/mL | ������� | | ��� | ��Ӧ�¶�/�� | ������� |
| 1 | 2.8 | �ࡢ��ɫ | | 1 | 40 | �ࡢ��ɫ |
| 2 | 2.4 | �ࡢ��ɫ | | 2 | 60 | �١�dz��ɫ |
| 3 | 2.0 | �϶ࡢ��ɫ | | 3 | 75 | �϶ࡢ��ɫ |
| 4 | 1.6 | ���١���ɫ | | 4 | 80 | �϶ࡢ��ɫ(������ɫ) |
��ʵ������ȡ������ȸʯ��Ӧ�ò��õ���������� ��
��80��ʱ�����ƵõĿ�ȸʯ��������ɫ���ʵĿ���ԭ���� ��
(2)ʵ��С��Ϊ�ⶨ����ij���������Ƶõļ�ʽ̼��ͭ��Ʒ��ɣ�������ͼ��ʾ��װ��(�г�����ʡ��)����ʵ�飺
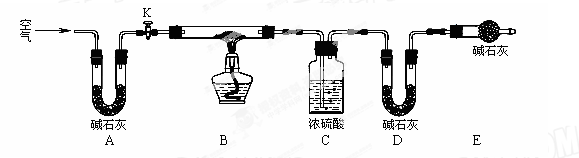
����1�����װ�õ������ԣ������ˡ�ϴ�Ӳ����������Ʒ����ƽֱ�������С�
����2������K�����������һ��ʱ���رգ��������װ�õ�������
����3������װ��Bֱ��װ��C�������ݲ�����
����4�� (�벹��ò���������)��
����5���������װ�õ�������
��װ��A�������� ������װ��E����ʵ��ⶨ��x/y��ֵ�� ��(ѡ�ƫ����ƫС������Ӱ�족)��
��ijͬѧ��ʵ������вɼ����������ݣ�
A����Ӧǰ����������Ʒ������163.8g B����Ӧ�������в�����������56.0g
C��װ��Cʵ�������9.0g D��װ��Dʵ�������8.8g
Ϊ�ⶨx/y��ֵ������Ϊ����ѡ���������ɼ������е� (д��������ϵ���ĸ����)��һ�鼴�ɽ��м��㣬��������ļ�������д������Ʒ��ɵĻ�ѧʽ ��
 12Na2CrO4+3Fe2O3+7KCl+12H2O
12Na2CrO4+3Fe2O3+7KCl+12H2O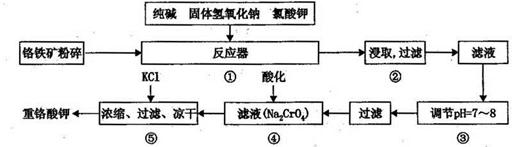

 ��100%�����м��㡣�ɴ˷�������5�еζ���Ӧ�����ӷ���ʽΪ ��
��100%�����м��㡣�ɴ˷�������5�еζ���Ӧ�����ӷ���ʽΪ ��