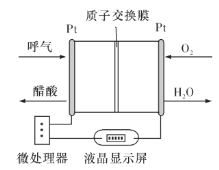
��Ŀ����
����Ŀ�������������һ�����Ƴ���������ҩ����ɰ�����(![]() )�ͼ״���Ӧ�Ƶá����������գ�
)�ͼ״���Ӧ�Ƶá����������գ�
(1)д������������Ľṹ��ʽ��_______________________________��
(2)��ҵ���� �����IJ���ͬ���͵ķ�Ӧ�Ƶð����ᡣ�밴ʵ�ʽ��еķ�Ӧ˳��д��ָ����Ӧ����������Ҫ���Լ��ͷ�Ӧ������
�����IJ���ͬ���͵ķ�Ӧ�Ƶð����ᡣ�밴ʵ�ʽ��еķ�Ӧ˳��д��ָ����Ӧ����������Ҫ���Լ��ͷ�Ӧ������
��һ��________________________________�� �ڶ���________________________________��
(3)д�����IJ���Ӧ�Ļ�ѧ����ʽ_______________________________________________��
(4)A�DZ� ������̼ԭ�ӵ�һ��ͬϵ���A������̼ԭ�ӿ��Դ���ͬһƽ���ϡ�д��A�Ľṹ��ʽ________________________________��
������̼ԭ�ӵ�һ��ͬϵ���A������̼ԭ�ӿ��Դ���ͬһƽ���ϡ�д��A�Ľṹ��ʽ________________________________��
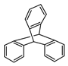
(5) ![]() �ǰ����������ij��ͬ���칹���һ�ȴ��������������������Һ���ȣ����ɵ��л�����Ľṹ��ʽΪ_____________________________________��
�ǰ����������ij��ͬ���칹���һ�ȴ��������������������Һ���ȣ����ɵ��л�����Ľṹ��ʽΪ_____________________________________��
���𰸡�![]() Br2��CCl4��Һ������ˮ�� NaOHˮ��Һ������
Br2��CCl4��Һ������ˮ�� NaOHˮ��Һ������ 
![]()
![]() +H2O
+H2O ![]()
![]() ��CH2=CH-COONa
��CH2=CH-COONa
��������
����������ǰ�����ͼ״�����������Ӧ�õ��ģ������Ậ��̼̼˫�����Ȼ����ںϳɹ�����Ҫע�Ᵽ��̼̼˫�����ݴ˻ش���Ŀ��
(1)�������������������ṹ��ʽΪ![]() ��
��
(2)��ҵ����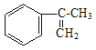 �����IJ���ͬ���͵ķ�Ӧ�Ƶð����ᣬ�ṹ�к���̼̼˫�����ʷ�ӦҪ��˫�����������ֳ������������Ʒ�ԭ������Ӧ���̿����ǰ���������ˮ��Ӧ������
�����IJ���ͬ���͵ķ�Ӧ�Ƶð����ᣬ�ṹ�к���̼̼˫�����ʷ�ӦҪ��˫�����������ֳ������������Ʒ�ԭ������Ӧ���̿����ǰ���������ˮ��Ӧ������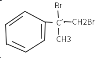 ������������ˮ��Һ��Ӧ����ԭ��ȡ��Ϊ�ǻ������������ص�ǿ��������Ӧ��-CH2OH����Ϊ�Ȼ������Ũ���ỷ��������ȥ��Ӧ��̼̼˫���ع顣��һ��ΪBr2��CCl4��Һ������ˮ���� �ڶ���NaOHˮ��Һ�����ȣ����IJ���Ӧ�Ļ�ѧ����ʽΪ
������������ˮ��Һ��Ӧ����ԭ��ȡ��Ϊ�ǻ������������ص�ǿ��������Ӧ��-CH2OH����Ϊ�Ȼ������Ũ���ỷ��������ȥ��Ӧ��̼̼˫���ع顣��һ��ΪBr2��CCl4��Һ������ˮ���� �ڶ���NaOHˮ��Һ�����ȣ����IJ���Ӧ�Ļ�ѧ����ʽΪ
![]()
![]() +H2O��
+H2O��
(4)AΪ ��һ��ͬϵ���A������̼ԭ�ӿ��Դ���ͬһƽ���ϣ�˵�������2��̼ԭ���벻���ͼ�������A�Ľṹ��ʽΪ
��һ��ͬϵ���A������̼ԭ�ӿ��Դ���ͬһƽ���ϣ�˵�������2��̼ԭ���벻���ͼ�������A�Ľṹ��ʽΪ![]() ��
��
(5) ![]() ����������������Һ���ȣ�̼�ȼ�����ˮ�ⷴӦ�������ǻ�����������ˮ�ⷴӦ�������ϲ����ķ��ǻ��������������Ʒ�Ӧ�����ɵ��л�����Ľṹ��ʽΪ
����������������Һ���ȣ�̼�ȼ�����ˮ�ⷴӦ�������ǻ�����������ˮ�ⷴӦ�������ϲ����ķ��ǻ��������������Ʒ�Ӧ�����ɵ��л�����Ľṹ��ʽΪ![]() ��CH2=CH-COONa��
��CH2=CH-COONa��

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�����Ŀ�������ٷɻ���ƽ�������ʱ��β���е�NO���ƻ������㡣��ѧ�������о����ô�������β���е�NO��COת���CO2��N2����ѧ����ʽ���£�2NO��2CO![]() 2CO2��N2��Ϊ�˲ⶨ��ij�ִ��������µķ�Ӧ���ʣ���ij�¶��������崫������ò�ͬʱ���NO��COŨ�������
2CO2��N2��Ϊ�˲ⶨ��ij�ִ��������µķ�Ӧ���ʣ���ij�¶��������崫������ò�ͬʱ���NO��COŨ�������
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
c(NO)/(10��4mol��L��1) | 10 | 4.5 | 2.5 | 1.5 | 1.0 | 1.0 |
c(CO)/(10��3mol��L��1) | 36.0 | 30.5 | 28.5 | 27.5 | 27.0 | 27.0 |
��ش���������(���������¶ȱ仯�Դ�����Ч�ʵ�Ӱ��)��
(1) ��֪�÷�Ӧ��S<0�������������·�Ӧ�ܹ��Է����У���Ӧ�Ħ�H________0(��д��>����<������)��
(2)ǰ2 s�ڵ�ƽ����Ӧ����v(N2)��________��
(3)�ڸ��¶��£���Ӧ��ƽ�ⳣ������ʽK��________��
(4)�������ܱ������з���������Ӧ���ﵽƽ��ʱ���д�ʩ�����NOת���ʵ���________��
A��ѡ�ø���Ч�Ĵ��� B�����߷�Ӧ��ϵ���¶�
C�����ͷ�Ӧ��ϵ���¶� D����С���������