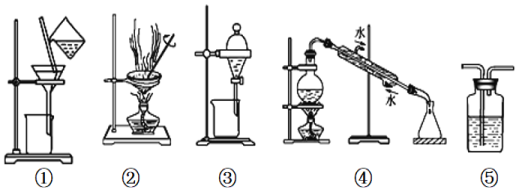
��Ŀ����
����Ŀ��ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ��
��1��ϡ����Ӧ����___________�У���д����������.
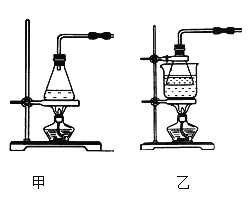
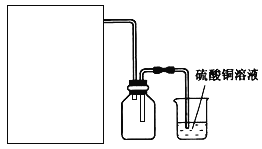
��2����ʵ��ͨ������A��B��C�����������������еĿ����ž����ٹرտ���______������_______�Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п���������_________________��
��3����ʵ��ʹ�����ۣ�����Ӧ���ʿ���̫���⣬�����ܻ���ɵIJ��������__________________��
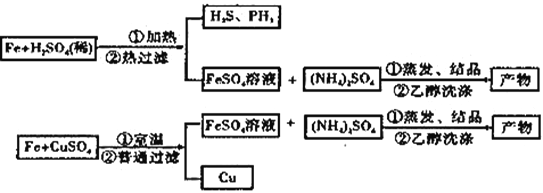
��4����FeSO4��Һ�м���(NH4��2SO4������Ʊ���������茶���[(NH4��2SO4��FeSO4��6H2O] ��ʽ��Ϊ392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ��(NH4��2SO4��FeSO4��6H2O�ֲ�Ʒ�����з���������ʵ���__________��
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol��L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�ζ������з�����Ӧ�����ӷ���ʽΪ______________________.�ζ��յ��������_____________________.ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ______________������ĸac�������ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������_______��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ�����Ũ�Ƚ���
���𰸡� ��Һ©�� B A ��ֹ���ɵ����������������� ���۽��뵼�ܴӶ��������� D MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O ���һ�ε�������Һ����ɫ��Ϊdz��ɫ����30s����ɫ ![]() ��100% BC
��100% BC
����������1��ʵ�鿪ʼ��ʱ��ϡ����Ӧ�÷��ڷ�Һ©���У�ʵ�����μӣ������۷�Ӧ��������������������
��2���ž������رտ���B����A��������Ӧ�������ɵ��������ݳ���ֻ����2�оۼ���ѹǿ����������Һ������Aѹ��װ��3�ڡ��ž�װ���ڵĿ�������ҪĿ����Ϊ���ų�������������������������������Ϊ����������
��3�����ۿ���̫С�����ܽ����ܿڶ�ס��
��4������Ϊ�þ���������ˮ���������Ҵ�������Ӧ��ѡ����90%���Ҵ���Һϴ���������Ա����Ʒ����ʧ��
�����Ը��������Һ��������������Ϊ�����ӣ����Ӧ����ʽΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O���ﵽ�ζ��յ��ʱ������Һ�����е��������Ӷ�����Ӧ������Ĺ����ĸ������ʹ��Һ��dz��ɫ�������յ�����Ϊ�����һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ����һ�ε�ʵ������̫��������ȥ�������ε�ƽ��ֵΪ25.00mL�����Ը������Ϊ25c/1000mol����������Ϊ��5����������c/8mol�����500mL��Һ��ȡ��25ml������ԭ��������Ϊ![]() ��������������茶���Ϊ5c/2 mol������Ϊ392��5c/2�����Բ�Ʒ����Ϊ��392��5c/2a=980c/a��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ㣬�Ὣ������ص������С������ѡ��A���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ��Ὣ����ĸ�����ص�������ѡ��B��ȷ����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ����ƿ�Dz���Ҫ��ϴ�ģ������ϴ�ˣ���ƿ�ڱ��ϻ���һ������������泥���������Ҫ�������ĸ��������Һ��ѡ��C��ȷ�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ���ô���εζ�����ʹ����ͬ�ĸ������Ũ�����Ӧ�ò����С��ѡ��D����
��������������茶���Ϊ5c/2 mol������Ϊ392��5c/2�����Բ�Ʒ����Ϊ��392��5c/2a=980c/a��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ㣬�Ὣ������ص������С������ѡ��A���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݣ��Ὣ����ĸ�����ص�������ѡ��B��ȷ����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ����ƿ�Dz���Ҫ��ϴ�ģ������ϴ�ˣ���ƿ�ڱ��ϻ���һ������������泥���������Ҫ�������ĸ��������Һ��ѡ��C��ȷ�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ���ô���εζ�����ʹ����ͬ�ĸ������Ũ�����Ӧ�ò����С��ѡ��D����