��Ŀ����
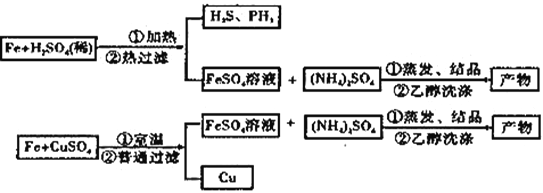
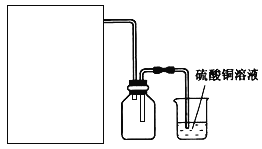
����Ŀ�����������[(NH4)2SO4��FeSO4��6H2O]�ֳ�Ħ���Ρ�ij��ȤС���Ʊ���������淋�ʵ�鷽��ʾ��ͼ���£�
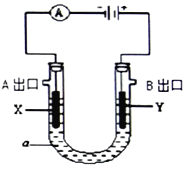
����A����ͼ������B����ͼ��
��֪��H2S��PH3Ϊ�ж����壬���ܱ�CuSO4��Һ���ճ�ȥ��
��ش��������⣺
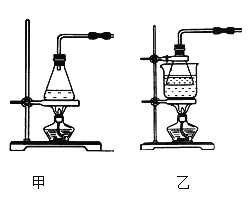
��1��ʵ��ǰ������н�����м����̼������Һ����У��㵹��Һ�壬��ˮϴ����м�IJ������衣������������ѡ����װ����ɸò��������������_________�����ţ���
������̨�ڲ������۹��ƿ��ʯ�������ձ���©���߾ƾ���
��2������A��FeSO4��Һ���Ʊ����������õ���м������ƿ�����������3 mol��L��1H2SO4��Һ����������ַ�ӦΪֹ�����ȹ��ˣ��ռ���Һ��ϴ��Һ������ͼװ���з�����ѡ����ʺ�����ʵ�鷽��A��װ����________����ס����ҡ�����
��3������A�з���м�к����������������Ʊ�ǰ��ȥ�������ǣ������ӷ���ʽ�ش�___________������ˮ�Ҵ�ϴ�Ӿ����ԭ����___________________��
��4��ʵ�鷽��B��FeSO4��Һ���Ʊ����á���ˮ����ͭ�ᾧˮ�IJⶨ��ʵ���еķ��������ˮ����ͭ��ĩ����ϡ��������м��Ӧ�Ʊ�����������������ˮ����ͭ���Ƶ���Һ����ɫ��ȫ��Ϊdz��ɫʱֹͣ��Ӧ�����ˣ��ռ���Һ��������������Һ���˷������ŵ���_______________�����ţ���
a.���������ʵ���ҷ����������ͭ��ĩ��������ҩƷ�˷ѣ��ֻ����˽���ͭ��ʵ���˱��Ϊ����
b.�������ж�������ŷŶԻ�����ɵ���Ⱦ��
c.ԭ�������ʴﵽ100%��
d.�������ڼ�����Fe2+��������Fe3+�� �����ڲ�Ʒ�����ȼ�����ߣ�����Լ����Դ��
��5����Ʒ��Fe3+���ʵĶ�������
������Fe3+Ũ��Ϊ1.0mg/mL�ı���Һ100mL����ȡ_______mg�ߴ��ȵ��������[(NH4)Fe(SO4)2��12H2O]����2.00 mL��������ȥ����ˮ�����ܽ����2mol��L��1HBr��Һl m L��l mol��L��1KSCN��Һ0.5 mL����ˮ�Ƴ�100 mL��Һ�����ƹ����б����õ��Ķ�������Ϊ____________��ѡ���ţ���
a.��ȷ��Ϊ0.001g�ĵ�����ƽb.��ȷ��Ϊ0.1mg�ĵ�����ƽ
c.100 mL����ƿd.��ʽ�ζ���e.10mL��Ͳ
�ڽ�������Һϡ��ΪŨ�ȷֱ�Ϊ0.2��1.0��3.0��5.0��7.0��10.0����λ��mg/L������Һ���ֱ�ⶨ��ͬŨ����Һ�Թ�����ճ̶ȣ������ⶨ������Ƴ��������¡�
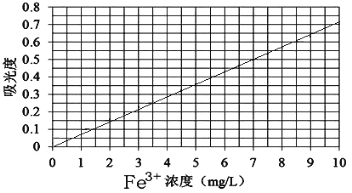
��ȡ����ȤС�����õ���������鱗�Ʒ�����������ò�Ʒ��Һ10mL��ϡ����100 mL��Ȼ����ڵķ������вⶨ�����βⶨ���õ�����ȷֱ�Ϊ0.490��0.510�������ȤС�������Ʒ�����������Һ������Fe3+Ũ��Ϊ_________mg/L��
���𰸡� �٢ڢܢݢ� �� Fe2O3+ 6H+= 2Fe3++ 3H2O��2Fe3++ Fe = 3Fe2+ ��������茶��岻�����Ҵ����Ҵ����Գ�ȥ��������ˮ�� abd 860.7 bcde 70
����������1��������м����̼������Һ����У���Ҫ�õ�����̨���ƾ��Ƽ��ȣ����ձ��н��У���Ҫ��ʯ��������ˮϴ���õ���м��Ҫ���˵IJ�������Ҫ©���Ͳ����������н�����м����̼������Һ����У��㵹��Һ�壬��ˮϴ����м�IJ������裬��ɸò�������������Т٢ڢܢݢߣ�
��2�������ҵ��������ڼ��ȵķ�ʽ��ͬ��Fe��ϡ���᳣�����ܹ�������Ӧ���ʵ������¶������ڷ�Ӧ�Ľ��У�ˮԡ���������ڿ����¶ȣ����Ʒ�Ӧ�����ʣ�����ѡ����װ�ã�
��3��Fe2O3 ��ϡ�����з�����Fe2O3 + 6H+ = 2Fe3+ + 3H2O�� 2Fe3+ + Fe = 3Fe2+�����Բ������Ʊ�ǰ��ȥFe2O3 ��Ϊ������������茶������ʧ��������������茶��岻�����Ҵ������Ҵ��ӷ����ӷ������д���ˮ�֣���ȥ��������ˮ����������ȷ�𰸣���������茶��岻�����Ҵ����Ҵ����Գ�ȥ��������ˮ�֣�
��4����Ϊ��Fe+CuSO4=Cu+FeSO4��Ӧ������ʵ�������÷����������ͭ��ĩ��Fe��Ӧ��ȡFeSO4��aѡ����ȷ���÷�Ӧ����Һ�н��У�û��H2S��PH3�ж������ŷţ�bѡ����ȷ��ԭ�������ʰٷ�֮�ٵ���˼�Ƿ�Ӧû�и��������ɣ����е�ԭ�Ӿ������ã����ø÷�����ȡFeSO4�Ĺ�������Cu���ɣ�����ԭ�������ʲ��ǰٷ�֮�٣�cѡ���������Fe�۹������������ڼ�����Fe2+��������Fe3+�������FeSO4�Ĵ��ȣ�dѡ����ȷ��������ʵ���еķ��������ˮ����ͭ��ĩ����ϡ��������м��Ӧ�Ʊ������������˷������ŵ��У�abd��
��5����������Fe3+Ũ��Ϊ1.0mg/mL�ı���Һ100mL��Ҫ�ߴ��ȵ��������xg��
1.0mg/mL��100mL��x=56:482��x=860.7mg���ڳ�����Ҫ�õ���ȷ��0.1mg�ĵ�����ƽ��ѡ����b������100ml��ҺҪ100ml����ƿ��ѡ����c����ȷ��ȡ2.00ml��ȥ����ˮ��10ml����Ͳ��ȷ�Ȳ�������Ҫ��ʽ�ζ��ܣ�ѡ����d�����ƹ����б����õ��Ķ�������Ϊbcde���ڸ���ͼ�����βⶨ���õ�����ȷֱ�Ϊ0.490��0.510���ӱ��п��Եõ�ʵ���õ������������Һ������Fe3+Ũ��Ϊ7mg/L����ϡ��10���Ժ����Һ�����������Ʒ�����������Һ������Fe3+Ũ��Ϊ70mg/��

 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�����Ŀ����NA����٤��������ֵ����֪��Ӧ
��1��CH4(g)+2O2(g)�TCO2(g)+2H2O(l) ��H1="a" kJ/mol
��2��CH4(g)+2O2(g)�TCO2(g)+2H2O(g) ��H2="b" kJ/mol���������������
��ѧ�� | C�TO | O�TO | C-H | O-H |
����kJ��mol-1 | 798 | x | 413 | 463 |
����˵����ȷ����
A. �ϱ��� x=(1796+b)/2
B. H2O(g)�TH2O(l) ��S��0����H�T(a-b )kJ/mol
C. ����4NA��C-H������ʱ���÷�Ӧ�ų�����һ��Ϊa kJ
D. ���÷�Ӧ��1����Ƶ�ԭ��ص�⾫��ͭʱ�����������0.2NA������ʱ�����۵���������һ������6.4g
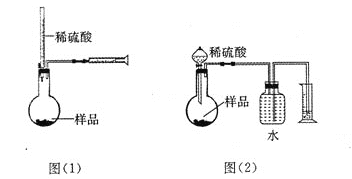
����Ŀ��ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ��
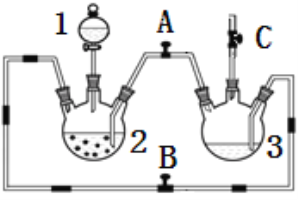
��1��ϡ����Ӧ����___________�У���д����������.
��2����ʵ��ͨ������A��B��C�����������������еĿ����ž����ٹرտ���______������_______�Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п���������_________________��
��3����ʵ��ʹ�����ۣ�����Ӧ���ʿ���̫���⣬�����ܻ���ɵIJ��������__________________��
��4����FeSO4��Һ�м���(NH4��2SO4������Ʊ���������茶���[(NH4��2SO4��FeSO4��6H2O] ��ʽ��Ϊ392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ��(NH4��2SO4��FeSO4��6H2O�ֲ�Ʒ�����з���������ʵ���__________��
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol��L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�ζ������з�����Ӧ�����ӷ���ʽΪ______________________.�ζ��յ��������_____________________.ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ______________������ĸac�������ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������_______��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ�����Ũ�Ƚ���