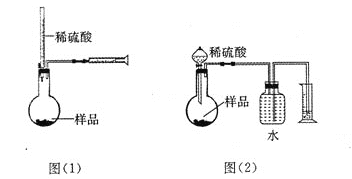
��Ŀ����
����Ŀ���������ⶨˮ���ܽ����ķ����ǣ�
����ȡa mLˮ����Ѹ�ټ���̶���MnSO4��Һ�ͼ���KI��Һ(��KOH)����������ƿ����������ʹ֮��ַ�Ӧ���䷴ӦʽΪ��2Mn2����O2��4OH��===2MnO(OH)2(�÷�Ӧ����)��
�ڲⶨ��������Ѹ�ټ���1��2 mLŨ����(�ữ���ṩH��)��ʹ֮����I2������b mol/L��Na2S2O3��Һ�ζ�(�Ե���Ϊָʾ��)������V mL���йط�ӦʽΪ��MnO(OH)2��2I����4H��===Mn2����I2��3H2O��I2��2S2O![]() ===2I����S4O
===2I����S4O![]() ��
��
�Իش�
��1���ζ��������õ��IJ�������������ʽ�ζ��ܡ���ʽ�ζ����ȱ��_____________��
��2���ζ�����ʱ�����ֿ��Ƶζ��ܣ�����________���۾�Ҫע��________��
��3��ˮ���ܽ����ļ���ʽ��______(��g/LΪ��λ)��
��4���ζ�(I2��S2O![]() ��Ӧ)�Ե���Ϊָʾ�����յ�ʱ��Һ��________ɫ��Ϊ________ɫ���Ұ�����ڲ���ɫ��
��Ӧ)�Ե���Ϊָʾ�����յ�ʱ��Һ��________ɫ��Ϊ________ɫ���Ұ�����ڲ���ɫ��
���𰸡� ��ƿ���ձ� ��������ƿ ��ƿ����Һ��ɫ�ı仯 8bV/a �� ��
�������������������1�������к͵ζ����������жϣ�
��2���ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���ߵα����۾�ע����ƿ����Һ��ɫ�ı仯��
��3�����ݹ�ϵʽ��O2��2MnO��OH��2��2I2��4S2O32-�ɼ���ˮ�����ܽ�����Ũ�ȣ���4������I2�ĵ�����Һ����ɫ������S2O32-����I2���յ�ʱI2��ȫ��Ӧ��
����������1���к͵ζ�ǰ����и����ݡ�����Ȳ��������ձ�ʢҺ�壬�ζ�������ƿʢ����Һ���õζ���ʢ��Һ��������ʽ�ζ��ܡ���ʽ�ζ����ȱ����ƿ���ձ�����2���ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���ߵα����۾�ע����ƿ����Һ��ɫ�ı仯����3��
���ݷ�Ӧ��2Mn2++O2+4OH-�T2MnO��OH��2�� MnO��OH��2+2I-+4H+�TMn2-+I2+3H2O��I2+2S2O32-�TS4O62-+2I-��
��֪��ϵʽ��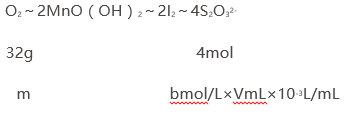
���m=8bV��10-3g����1Lˮ������������Ϊ�� ![]() g/L
g/L
��4������I2�ĵ�����Һ����ɫ������S2O32-����I2���յ�ʱI2��ȫ��Ӧ���յ�ʱ��Һ����ɫɫ��Ϊ��ɫɫ���Ұ�����ڲ���ɫ��

����Ŀ����NA����٤��������ֵ����֪��Ӧ
��1��CH4(g)+2O2(g)�TCO2(g)+2H2O(l) ��H1="a" kJ/mol
��2��CH4(g)+2O2(g)�TCO2(g)+2H2O(g) ��H2="b" kJ/mol���������������
��ѧ�� | C�TO | O�TO | C-H | O-H |
����kJ��mol-1 | 798 | x | 413 | 463 |
����˵����ȷ����
A. �ϱ��� x=(1796+b)/2
B. H2O(g)�TH2O(l) ��S��0����H�T(a-b )kJ/mol
C. ����4NA��C-H������ʱ���÷�Ӧ�ų�����һ��Ϊa kJ
D. ���÷�Ӧ��1����Ƶ�ԭ��ص�⾫��ͭʱ�����������0.2NA������ʱ�����۵���������һ������6.4g
����Ŀ��ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ��
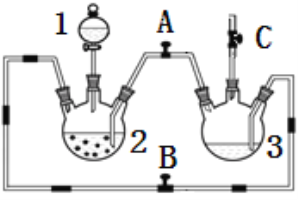
��1��ϡ����Ӧ����___________�У���д����������.
��2����ʵ��ͨ������A��B��C�����������������еĿ����ž����ٹرտ���______������_______�Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п���������_________________��
��3����ʵ��ʹ�����ۣ�����Ӧ���ʿ���̫���⣬�����ܻ���ɵIJ��������__________________��
��4����FeSO4��Һ�м���(NH4��2SO4������Ʊ���������茶���[(NH4��2SO4��FeSO4��6H2O] ��ʽ��Ϊ392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ��(NH4��2SO4��FeSO4��6H2O�ֲ�Ʒ�����з���������ʵ���__________��
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol��L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�ζ������з�����Ӧ�����ӷ���ʽΪ______________________.�ζ��յ��������_____________________.ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ______________������ĸac�������ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������_______��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ�����Ũ�Ƚ���