��Ŀ����
13���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺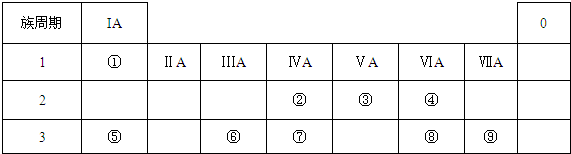
��1������Ԫ�آ�����ӽṹʾ��ͼ

��2���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��ΪNa��Al��O����Ԫ�ط��ű�ʾ����
��3���ڡ��ߡ������ۺ������������ǿ������˳��ΪH2SO4��H2CO3��H2SiO3��
��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ
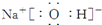 ��
�� 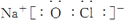 ��
����5��Ԫ�آܵ�һ���⻯��A��һ�������·ֽ�Ϊ������һ���⻯��B��д��A�ĽṹʽH-O-O-H��д��A��Fe2+��ǿ���������·�Ӧ�����ӷ���ʽH2O2+2Fe2++2H+=2Fe3++2H2O��
��6�������Т٢ܢݢ�����Ԫ�ص����ֻ������ˮ��Һ��Ͽ��Է�����Ӧ��д�����ӷ���ʽHSO3-+H+=SO2��+H2O��
���� ����Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪS����ΪCl��
��1��S��Ӧ�����Ӻ�����3�����Ӳ㣬����������Ϊ8��
��2�����Ӳ�Խ��뾶Խ���Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС��
��3��Ԫ�صķǽ�����Խǿ����Ӧ�ĸ��������Ӧˮ���������Խǿ��
��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γ�NaOH��NaClO�����ӻ����
��5��Ԫ�آ�ΪO��O��һ���⻯��A��һ�������·ֽ�Ϊ������һ���⻯��B����AΪH2O2��BΪH2O��H2O2����ǿ�����ԣ�
��6�������Т٢ܢݢ�����Ԫ�ص����ֻ����ﳣ��ΪNaHSO3��NaHSO4�����߷�Ӧ�����ɶ��������ˮ��
��� �⣺����Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪSi����ΪS����ΪCl��
��1����ΪS����Ӧ�����Ӻ�����3�����Ӳ㣬����������Ϊ8���ṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2�����Ӳ�Խ��뾶Խ������Na��Al��O�����Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС����Na��Al���ʴ�Ϊ��Na��Al��O��
��3���ڢ���C��Si����ͬ����Ԫ�أ�����Ԫ�������ɣ����ϵ�������������Ӧˮ���������������H2CO3��H2SiO3����ΪS���ǽ���S��C��Ԫ�صķǽ�����Խǿ����Ӧ�ĸ��������Ӧˮ���������Խǿ���������ԣ�H2SO4��H2CO3��
�ʴ�Ϊ��H2SO4��H2CO3��H2SiO3��
��4���١��ܡ��ݡ����е�ijЩԪ�ؿ��γ�NaOH��NaClO�����ӻ��������ʽ�ֱ�Ϊ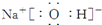 ��
��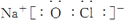 ��
��
�ʴ�Ϊ��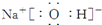 ��
�� 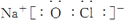 ��
��
��5��Ԫ�آ�ΪO��O��һ���⻯��A��һ�������·ֽ�Ϊ������һ���⻯��B����AΪH2O2������-O-O-��������ǿ�����ԣ�BΪH2O��H2O2�ĽṹʽΪH-O-O-H��H2O2��Fe2+�����������·�Ӧ���������ӡ�ˮ����Ӧ�����ӷ���ʽΪ��H2O2+2Fe2++2H+=2Fe3++2H2O��
�ʴ�Ϊ��H-O-O-H��H2O2+2Fe2++2H+=2Fe3++2H2O��
��6�������Т٢ܢݢ�����Ԫ�ص����ֻ����ﳣ��ΪNaHSO3��NaHSO4�����߷�Ӧ�����ɶ��������ˮ����Ӧ�����ӷ���ʽΪHSO3-+H+=SO2��+H2O��
�ʴ�Ϊ��HSO3-+H+=SO2��+H2O��
���� ���⿼��Ԫ�ص��ƶϣ���Ŀ�ѶȲ�����Ԫ�������ڱ��е����ʿ��ƶϳ�Ԫ�ص����࣬���в����������ɵ�Ӧ�ã�ѧϰ��ע��������֪ʶ�����յ���ʽ����д��

| Ԫ�� | �����Ϣ |
| A | ԭ�Ӻ���L���������K���2�� |
| C | ������ͬ�������壬����һ�ֿ������������к��Ķ̲������ߣ���ֹ�䵽�������� |
| D | �۵����Ų�Ϊ��n+1��s+��n+1��p��n+2�� |
| E | ��������ij�ּ�̬����������ɫ |
��1��B��Ԫ�����ڱ���λ�õڶ�����VA�壻E2+�ĺ�������Ų�ʽ��1s22s22p63s23p63d6��
��2��A��B��C�ĵ�һ�������ɴ�С��˳��ΪN��O��C��A��B��C�����������ȶ����ɴ�С��˳��Ϊ����д��ѧʽ����ͬ��H2O��NH3��CH4��B��C��D�⻯��ķе���ߵ�ΪH2O��
��3��1molAD2�����к��еĦм���ĿΪ2NA���û�����ľ��������Ƿ��Ӿ��壮
��4��������E������NaCl��Һ�з�����ʴʱ��������ӦʽΪO2+2H2O+4e-=4OH-��
��F2��H2O ��Na��H2O ��Na2O2��H2O ��P2O5��H2O ��Na2O��H2O
��Cl2��H2O ��NO2��H2O ��Al��NaOH��Һ��
| A�� | �ۢܢݢޢ� | B�� | �٢ݢ� | C�� | �ڢۢݢ� | D�� | �ۢޢ� |
| A�� | X����ۺ�����ķ���ʽ�ɱ�ʾΪH3XO4 | |
| B�� | X�ǵڶ�����VA��Ԫ�� | |
| C�� | X�ǵ������ڢ�A��Ԫ�� | |
| D�� | X������ϼ�Ϊ+4 |
��1�����NaCl��Һ����Cl2�Ļ�ѧ����ʽ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2��
��2����Cl2����H2O��NaOH��Һ�������ˮ��Ư��Һ��
�������������Ư�����ʣ�����ˮȴ��Ư�����ã�˵����Ư�����õ�������HClO
��25�棬Cl2��H2O��NaOH�ķ�Ӧ���£�
| ��Ӧ�� | Cl2+H2O?Cl-+H++HClO K1=4.5��10-4 |
| ��Ӧ�� | Cl2+2OH-?Cl-+ClO-+H2O K2=7.5��1015 |
��3����ͥʹ��Ư��Һʱ������ֱ�ӽӴ�����Ʒ��Ư��Һ��ʴ���ĵ缫��ӦΪ��Fe-2e-=Fe2+��ClO-�����ĵ缫��Ӧʽ��ClO-+2e-+H2O=Cl-+2OH-
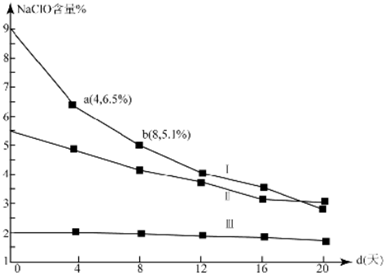
��4���о�Ư��Һ���ȶ��Զ��������ͱ�����ʵ�����壮30��ʱ��pH=11��Ư��Һ��NaClO�������ٷֺ�����ʱ��仯���£�
�ٷֽ���v����v���Ĵ�С��ϵ�ǣ���ԭ��������ͬ�����£��������Ƶ�Ũ��Խ����ֽ�����Խ��
��NaClO�ֽ�Ļ�ѧ����ʽ��2NaClO$\frac{\underline{\;30��\;}}{\;}$2NaCl+O2��
��4d��8d������v��NaClO��=0.047mol/��L��d����������Ư��Һ���ܶ�ԼΪ1g/cm3���ұ仯���Բ��ƣ�
| A�� | PCl3�����������Σ�������Ϊ��ԭ����sp2�ӻ��Ľ�� | |
| B�� | ��ϩ�����е�̼�������ԭ�ӵ�1s�����̼ԭ�ӵ�һ����sp3�ӻ�����γɵ� | |
| C�� | ����ԭ�Ӳ�ȡsp3�ӻ��ķ��ӣ��伸�ι��Ϳ������������λ������λ�V�� | |
| D�� | AB3�͵ķ��ӿռ乹�ͱ�Ϊƽ�������� |
| A�� | ʯ���ѽ�õ��������Ǵ����� | |
| B�� | ʯ�Ͳ�Ʒ�������ھۺϷ�Ӧ | |
| C�� | ��Ȼ����һ�����Ļ�ʯȼ�� | |
| D�� | ˮú����ͨ��ú��Һ���õ�������ȼ�� |
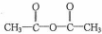 ��
��
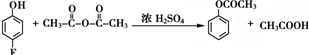 ����Ӧ�ݵĻ�ѧ����ʽΪ
����Ӧ�ݵĻ�ѧ����ʽΪ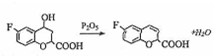 ��
��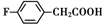 ��
��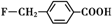 ��
�� ��������Ϊԭ���Ʊ���
��������Ϊԭ���Ʊ���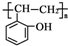 ���ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
���ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�