��Ŀ����
����Ŀ���״���һ�ֿ�������Դ����CO2�Ʊ��״��Ĺ��̿����漰�ķ�Ӧ���£�
��Ӧ����CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=��49.58 kJmol��1
CH3OH(g)+H2O(g) ��H1=��49.58 kJmol��1
��Ӧ����CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2
CO(g)+H2O(g) ��H2
��Ӧ����CO(g)+2H2(g)![]() CH3OH(g) ��H3=��90.77 kJmol��1
CH3OH(g) ��H3=��90.77 kJmol��1
�ش��������⣺
(1)��Ӧ��ġ�H2=_________������Ӧ����������ƽ�ⳣ���ֱ�ΪK1��K2��K3����K2=________(��K1��K3��ʾ)��
(2)��Ӧ���Է�����������___________(�����ϵ��¶��������ϸ��¶��������κ��¶���)��
(3)��һ��������2 L�����ܱ������г���3 mol H2��1.5 mol CO2����������Ӧ��ʵ���ò�ͬ��Ӧ�¶�����ϵ��CO2��ƽ��ת���ʵĹ�ϵ�����±���ʾ��
�¶�(��) | 500 | T |
CO2��ƽ��ת���� | 60�G | 40�G |
��T______500��(����>������<�� ����=��)��
���¶�Ϊ500��ʱ���÷�Ӧ10 minʱ�ﵽƽ�⡣��H2��ʾ�÷�Ӧ�ķ�Ӧ����v(H2)=______________�����¶��£���ӦI��ƽ�ⳣ��K=______________L2/mol2
(4)��CO2�Ʊ��״�����Ҫ��������ҵ���õ�ⷨ��ȡNa2FeO4��ͬʱ���������Fe+2H2O+2OH![]() FeO42+3H2��������ԭ����ͼ��ʾ�����һ��ʱ���c(OH)���͵�������__________ (������������������������)�����ҷ����ĵ缫��ӦʽΪ��___________________________��
FeO42+3H2��������ԭ����ͼ��ʾ�����һ��ʱ���c(OH)���͵�������__________ (������������������������)�����ҷ����ĵ缫��ӦʽΪ��___________________________��

���𰸡�+41.19 kJmol��1 K1/ K3 �ϵ��¶� > 0.135 mol��L��1��min��1 200 ������ Fe - 6e����8OH�� = FeO42����4H2O
��������
��1�����ݸ�˹���ɣ���=��-�÷�Ӧ��ġ�H2����Ӧ���Ϊ��Ӧ��͢�IJ���Է�Ӧ��ƽ�ⳣ��Ϊ��K2=K1/K3��
��2����ѧ��Ӧ�ܷ��Է����У�ȡ�����ʱ���ر���ۺ��оݣ�����G= ��H -T ��S![]() 0ʱ����Ӧ���Է����У��ݴ˽��
0ʱ����Ӧ���Է����У��ݴ˽��
��3���ٷ�Ӧ�������Ӧ�Ƿ��ȷ�Ӧ�����������¶ȶ�����̼��ת���ʼ�С��
�ڸ��ݦ�=![]() ���㻯ѧ��Ӧ���ʣ�ƽ�ⳣ������ƽ��ʱ������Ũ����֮���뷴Ӧ��Ũ����֮���ı�ֵ��
���㻯ѧ��Ӧ���ʣ�ƽ�ⳣ������ƽ��ʱ������Ũ����֮���뷴Ӧ��Ũ����֮���ı�ֵ��
��4����װ��Ϊ���أ������缫������Fe����������ʧȥ���ӣ������ĵ缫��ӦΪ��Fe - 6e����8OH�� = FeO42����4H2O������������OH-��
��1�����������ķ�Ӧ��CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H1=��49.58 kJmol��1
��Ӧ��CO2(g)+H2(g) CO(g)+H2O(g) ��H2
��Ӧ��CO(g)+2H2(g) CH3OH(g) ��H3=��90.77 kJmol��1
���ݸ�˹���ɿɵã���=��-��![]() H2=��H1- ��H3=��49.58 kJmol��1+90.77 kJmol��1=+41.19 kJmol��1����Ӧ���Ϊ��Ӧ��͢�IJ���Է�Ӧ��ƽ�ⳣ��Ϊ��K2=K1/K3��
H2=��H1- ��H3=��49.58 kJmol��1+90.77 kJmol��1=+41.19 kJmol��1����Ӧ���Ϊ��Ӧ��͢�IJ���Է�Ӧ��ƽ�ⳣ��Ϊ��K2=K1/K3��
�������+41.19 kJmol��1��K2=K1/K3.
��2������G= ��H -T ��S![]() 0ʱ����Ӧ�ܹ��Է����У��÷�Ӧ�ġ�S
0ʱ����Ӧ�ܹ��Է����У��÷�Ӧ�ġ�S![]() 0����H
0����H![]() 0�����¶Ƚϵ�ʱ����Ӧ���ܹ��Է����У�
0�����¶Ƚϵ�ʱ����Ӧ���ܹ��Է����У�
�����Ϊ���ϵ��¶ȡ�
��3���ٷ�Ӧ�������Ӧ�Ƿ��ȷ�Ӧ�����������¶ȶ�����̼��ת���ʼ�С�����¶�Խ��ת����Խ��T ����500�棻
�������>��
��CO2�ı仯��Ϊ1.5mol��60%=0.9mol
CO2(g)![]() 3H2(g) CH3OH(g)
3H2(g) CH3OH(g)![]() H2O(g)
H2O(g)
��ʼ��:1.5mol 3mol 0 0
�仯��:0.9mol 2.7mol 0.9mol 0.9mol
ƽ����:0.6mol 0.3mol 0.9mol 0.9mol
��(H2)=(2.7mol��2L)��10min=0.135 mol/L-1min-1��K= =200 L2/mol2
=200 L2/mol2
�������0.135molL-1min-1��200��
��4����װ��Ϊ���أ������缫������Fe����������ʧȥ���ӣ������ĵ缫��ӦΪ��Fe - 6e����8OH�� = FeO42����4H2O����������������OH-���������ŵ��Ľ���c(OH)�����ͣ�
������������� ��Fe - 6e����8OH�� = FeO42����4H2O��

����Ŀ����ҵ�����������������Ⱦ���������·�Ӧ��
CH4(g)��2NO2(g)![]() N2(g)��CO2(g)��2H2O(g) ��H��a kJ/mol
N2(g)��CO2(g)��2H2O(g) ��H��a kJ/mol
���¶�T1��T2ʱ���ֱ�0.50 mol CH4��1.2 mol NO2�������Ϊ1 L���ܱ������У����n(CH4)��ʱ��仯�������±���
�¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
T1 | n(CH4) | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
T2 | n(CH4) | 0.50 | 0.30 | 0.18 | ���� | 0.15 |
����˵������ȷ����
A. 10 min�ڣ�T1ʱv(CH4)T2ʱС B. �¶ȣ�T1��T2
C. ��H��a < 0 D. ƽ�ⳣ����K(T1)��K(T2)
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL 0.25mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL 0.55mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
����NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ������¶ȡ�
�ش��������⣺
��1������A������Ϊ_________________________��
��2������NaOH��Һ����ȷ������________��
A���ز����������� B���������������� C��һ��Ѹ�ٵ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������________��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β���������ؽ���
��4��ʵ���������±���
������д�±��еĿհף�
�¶� ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ �¶� t2/�� | �¶Ȳ� ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | _______ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�����Ƶ���Ϊ0.55mol/L NaOH��Һ��0.25mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H �� ______ (ȡС�����һλ)��
���к��Ȳⶨʵ���У����в���һ���ή��ʵ��ȷ�Ե���________��
A���õζ���(��������������������0.01)ȡ���������Һ�����
B��NaOH��Һ�ڵ���С�ձ�ʱ������������
C����С�ձ�������ϴв��ŵ�����ĭ���Ͻ϶�
D������HCl��Һ���¶ȼ���ˮϴ�����������NaOH��Һ���¶�
����Ŀ���ϳɰ������Ĵ����������˹��̵�����Ҫ;�������о�������ȷ������ָ�����ϳɰ���Ӧ��ƽ�ⳣ��Kֵ���¶ȵĹ�ϵ���£�
�¶ȣ��棩 | 360 | 440 | 520 |
Kֵ | 0.036 | 0.010 | 0.0038 |
��1����д����ҵ�ϳɰ��Ļ�ѧ����ʽ_________��
�����ϱ����ݿ�֪�÷�ӦΪ���ȷ�Ӧ��������_________��
�������ϣ�Ϊ������ƽ��ʱH2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_________��������ţ�
a. ����ѹǿ b. ʹ�ú��ʵĴ���
c. �����¶� d. ��ʱ����������е�NH3
��2��ԭ����H2��ͨ����ӦCH4(g)+H2O(g)![]() CO(g)+3H2(g)��ȡ����֪�÷�Ӧ�У�����ʼ������е�
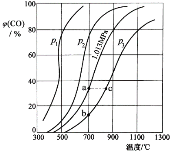
CO(g)+3H2(g)��ȡ����֪�÷�Ӧ�У�����ʼ������е�![]() �㶨ʱ���¶ȡ�ѹǿ��ƽ������CH4������Ӱ������ͼ��ʾ��
�㶨ʱ���¶ȡ�ѹǿ��ƽ������CH4������Ӱ������ͼ��ʾ��

��ͼ�У��������߱�ʾѹǿ�Ĺ�ϵ�ǣ�P1_____P2��������������=��������������
�ڸ÷�ӦΪ________��Ӧ��������������������������
��3��ԭ����H2����ͨ����ӦCH4(g)+H2O(g)![]() CO(g)+3H2(g)��ȡ��T��ʱ�����ݻ��̶�Ϊ5L�������г���1molˮ������1mol CO����Ӧ��ƽ����CO��Ũ��Ϊ0.08 mol��L-1
CO(g)+3H2(g)��ȡ��T��ʱ�����ݻ��̶�Ϊ5L�������г���1molˮ������1mol CO����Ӧ��ƽ����CO��Ũ��Ϊ0.08 mol��L-1
��ƽ��ʱCO��ת����Ϊ_________��
�ڸ��¶��·�Ӧ��ƽ�ⳣ��KֵΪ_________��