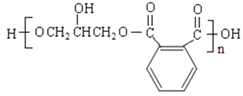
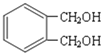
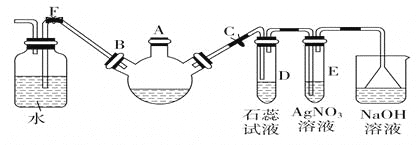
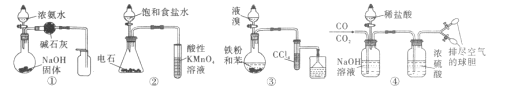
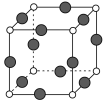
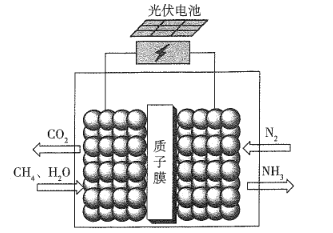
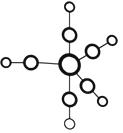
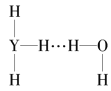��Ŀ����
����Ŀ���ס�����ͬѧ��һ�ֱ�����ȥ�ⶨͬһ��δ֪Ũ�ȵ�NaOH��Һ��Ũ�ȣ���������ͬ���װ�һ�������NaOH��Һ������ƿ���ѱ��������ζ��ܽ��еζ����Ұ�һ������ı����������ƿ����δ֪ҺNaOH��Һ����ζ��ܽ��еζ���
(1)��ͬѧʹ�õ���_______�ζ��ܣ���ͬѧʹ�õ���________�ζ��ܡ�
(2)��ͬѧ�ĵζ�����������ˮϴ����û���ñ�������ϴ����ͬѧ�ĵζ�����������ˮϴ����Ҳû���ô���NaOH��Һ��ϴ�������������ȷ��������ͬѧ�ⶨ���________(ƫ��ƫС����Ӱ�죬��ͬ)����ͬѧ�ⶨ���__________��
(3)��ͬѧѡ���̪��ָʾ���������飬������жϵζ��յ㣺____________��
(4)��ͬѧ��������ʵ��ֱ��¼�й��������£�
�ζ����� | ��������������Һ�����/mL | 0.1000mol/L����������mL�� | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ��/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
��ѡ�����к��������ݼ���c(NaOH)=______________��
���𰸡���ʽ ��ʽ ƫ�� ƫС �������һ��NaOH��Һ����ƿ����Һ����ɫ��Ϊ�ۺ�ɫ����30���ڲ���ɫ 0.1044mol/L
��������
(1)�к͵ζ�ʱ������Һ������ʽ�ζ����У�����Һ���ڼ�ʽ�ζ����У�
(2)��ͬѧ�ĵζ�����������ˮϴ����û���ñ�������ϴ�����±�ҺŨ��ƫ�ͣ���ͬѧ�ĵζ�����������ˮϴ����Ҳû���ô���NaOH��Һ��ϴ�����´���ҺŨ��ƫ�ͣ�
(3)�ζ��յ�ʱ��ƿ����Һ����ɫ��Ϊ�ۺ�ɫ����30���ڲ���ɫ��
(4)��ȥ���ϴ�ڶ���ʵ�����ݣ�����n(NaOH) ��V(NaOH)=n(HCl)��V(HCl)���㡣
(1)�к͵ζ�ʱ��������Һ������ʽ�ζ����У�����������Һ���ڼ�ʽ�ζ����С����Լ�ͬѧʹ�õ�����ʽ�ζ��ܣ���ͬѧʹ�õ��Ǽ�ʽ�ζ��ܣ�
(2)��ͬѧ�ĵζ�����������ˮϴ����û���ñ�������ϴ�����±�ҺŨ��ƫ�ͣ�����Ҫ��Һ���ƫ��ʹ�������ƫ����ͬѧ�ĵζ�����������ˮϴ����Ҳû���ô���NaOH��Һ��ϴ�����´���ҺŨ��ƫ�ͣ�����Ҫ�����������ƫ���������Ƶ�Ũ��ƫ�ͣ�
(3)�к͵ζ�ʱ����ͬѧ������������Һ�μ����ᣬָʾ����̪��������Һ�У���ʼ��ɫ������NaOH�ĵ��룬��Һ������������������ǿ�����������һ��NaOH��Һ������ƿ����Һ����ɫ��Ϊ�ۺ�ɫ����30���ڲ���ɫ����֤���ﵽ�ζ��յ㣻
(4)���ݱ������ݿ�֪���ڶ���ʵ�����ϴ�Ӧ��ȥ������Ҫ��������ΪV=![]() mL=26.10mL�����ݶ���ǡ�÷�Ӧʱn(NaOH)=n(HCl)��֪��c(NaOH)��25.00��10-3L=0.1000mol/L��26.10��10-3L������c(NaOH)=0.1044mol/L��
mL=26.10mL�����ݶ���ǡ�÷�Ӧʱn(NaOH)=n(HCl)��֪��c(NaOH)��25.00��10-3L=0.1000mol/L��26.10��10-3L������c(NaOH)=0.1044mol/L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�