��Ŀ����
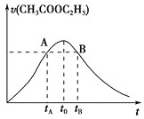
����Ŀ��ijʵ��С����Ƶ��dz����������÷�������ͼ��ʾ�����У�A��ֲ���������ڼ���B�Ǹ߷��ӻ����D�Ǿ���ˮ����ζ�����ʡ���ش��������⣺
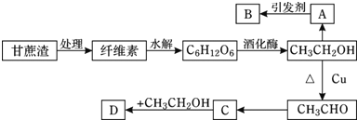
(1)��ά�صĻ�ѧʽΪ________����________(�����������������������)��
(2)B�Ľṹ��ʽΪ________��C�й����ŵ�����Ϊ________��
(3)д������ת���Ļ�ѧ����ʽ��CH3CH2OH��CH3CHO��_______����Ӧ����Ϊ________��
(4)��ѧʽΪC5H10O2��ͬ���칹������C��ͬϵ�����______�֣������ں˴Ź���������ֻ��2���壬�ҷ����֮��Ϊ1:9�Ľṹ��ʽΪ_______��
(5)����˵����ȷ����________(����ĸ)��
A.����A����Ҫ������ʯ���ѽ⣬������Ǻ���һ������ʯ�ͻ���ˮƽ�ı�־֮һ
B.����ʯ�͵õ��ĸ������Ϊ������
C.B����������ʳƷ��װ��
D.���ϡ��ϳ���ά���ϳ��ȶ��Ǻϳ��л��߷���
���𰸡�(C6H10O5)n ����� ![]() �Ȼ� 2CH3CH2OH+O2
�Ȼ� 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ������Ӧ 4 (CH3)3C-COOH ACD
2CH3CHO+2H2O ������Ӧ 4 (CH3)3C-COOH ACD
��������
�Ҵ���������A��A��ֲ���������ڼ�������AΪCH2=CH2��B�Ǹ߷��ӻ��������BΪ![]() ���Ҵ���������������ȩ����ȩ����������������CΪCH3COOH��������Ҵ����Է���������Ӧ���ɾ���ˮ����ζ��CH3CH2OOCH3��
���Ҵ���������������ȩ����ȩ����������������CΪCH3COOH��������Ҵ����Է���������Ӧ���ɾ���ˮ����ζ��CH3CH2OOCH3��
(1)��ά�صĻ�ѧʽΪ(C6H10O5)n������ÿ����ά�ط�������n����ֵ���ܲ�ͬ��������ά���ǻ���
(2)BΪ![]() ��CΪCH3COOH���������Ϊ�Ȼ���
��CΪCH3COOH���������Ϊ�Ȼ���
(3)�Ҵ���������������ȩ����ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O���÷�Ӧ����������Ӧ��
2CH3CHO+2H2O���÷�Ӧ����������Ӧ��
(4)��ѧʽΪC5H10O2����ͬ���칹������C��ͬϵ������Ȼ�������COOH����һ����C4H9��������4�֣�����ͬ���칹����4�֣������ں˴Ź���������ֻ��2���壬�ҷ����֮��Ϊ1:9�Ľṹ��ʽΪ(CH3)3C-COOH��
(5)A����ϩ����Ҫ��Դ��ʯ���ѽ⣬������Ǻ���һ������ʯ�ͻ���ˮƽ�ı�־֮һ����A��ȷ��
B������ʯ�͵õ��ĸ���������Ƕ�������Ļ�����B����
C������ϩ�����ȶ���������������������ʳƷ��װ������C��ȷ��
D�����ϡ��ϳ���ά���ϳ��ȶ����˹��ϳɵ��л��߷��ӻ����D��ȷ��
��������ѡACD��

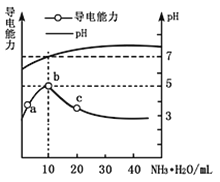
����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ� ������Cr��III���Ĵ��������������¡�
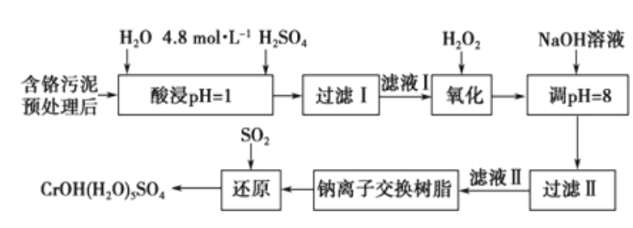
��֪���������ȡ����Һ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
��CrOH��H2O��5SO4������ˮ��
��1��ŨH2O2��Һ�������ǽ���ҺI�е�Cr3+ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ:____________��
��2�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
��ʼ����ʱ��pH | 2.7 | - | - | - |
������ȫʱ��pH | 3.7 | 11.1 | 5.2 | 5.6 |
����NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72-ת��ΪCr2O42-�������ӷ���ʽΪ______________________���˲�������Һ��pH����8��ͨ��������ȥ�Ľ�������Ϊ___________���˲�����û�м������߽�Mg2+��ȥ��������______________________��
��3�������ӽ�����֬�ķ�Ӧԭ��ΪMn++nNaR��MRn+nNa+�����ø÷�����Ҫ��ȥ����ҺII�е�Mg2+�� �������ҺIIͨ�������ӽ���������Һ��c��Na+��Ϊamol/L������ҺII��c��Mg2+��Ϊ___________��
��4��д��������������SO2���л�ԭʱ������Ӧ�����ӷ���ʽ��_________________________________��
����Ŀ���йض���������Ԫ��A��B��C��D��E��F����Ϣ���£�
�й���Ϣ | |
A | ����һ�ֺ��ص�������Ϊ0 |
B | ����������Ӧ��ˮ���ﰴ1��1��ȫ�������������ȵ����������� |
C | �ڵؿ��еĺ���Ϊ����λ |
D | �䵥����O2��ȼ�գ���������������ɫ���� |
E | ���γɻ�������������Ԫ�� |
F | �䵥�ʳ������������� |
(1)CԪ����________����Ԫ������)��D��Ԫ�����ڱ��е�λ����________��
(2)��ҵ�ϵõ�C�ĵ��ʳ����ö��Ե缫�������______���ѧʽ)�ķ�����C�ĵ��ʿ��Ժ�B������������ˮ�������Ӧ��д���÷�Ӧ�����ӷ���ʽ��______��C�γ�ij�ֻ�����LiCA4���ǽ���������������л��ϳ��еij����Լ�����ˮ���ͷų�A2����ôLiCA4��AԪ�صĻ��ϼ�Ϊ_________��
(3)��һ�������£�A��F���γ�һ��������ˮ��Һ��F2A4�������ʽΪ________�������ʿ���NaClO��FA3��Ӧ�Ƶã�д����Ӧ�Ļ�ѧ����ʽ��_______��ÿ����1mol F2A4ת�Ƶ�����Ϊ________��
(4)д��һ����֤��E��F�ǽ�����ǿ���Ļ�ѧ����ʽ________��
(5)������W��A��D��E��F����Ԫ����ɡ���W��Һ�е���FeCl3��Һ����Һ��Ѫ��ɫ����ȡһ��W��Һ����ǿ����Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ���������壬W�Ļ�ѧʽΪ________��
(6)F�ļ��⻯��ķе������ͬ����������Ԫ���⻯��е�ߣ�������__________��
����Ŀ�����ٻ����������ϣ��״�����ķ�Ӧ������ͼ��ʾ�������������ٴ��������ϵ�������*��ע��
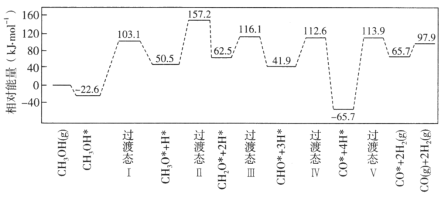
��1��![]() �ġ�H=_____________kJ��mol��1�����������������(���)E��=____________kJ��mol��1��д���ò���Ļ�ѧ����ʽ_________________��
�ġ�H=_____________kJ��mol��1�����������������(���)E��=____________kJ��mol��1��д���ò���Ļ�ѧ����ʽ_________________��
��2����һ���¶��£�CO��H2������巢����Ӧ��![]() ����Ӧ������=����������=k��c(CO)��c2(H2)��k��c(CH3OH)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ������ﵽƽ����������Ч������
����Ӧ������=����������=k��c(CO)��c2(H2)��k��c(CH3OH)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ������ﵽƽ����������Ч������![]() ��___________(����������������С������������)���������¶ȣ�
��___________(����������������С������������)���������¶ȣ�![]() ��____________(����������������С������������)��
��____________(����������������������������)��
��3��353Kʱ���ڸ��������г���CH3OH(g)��������Ӧ��![]() ����ϵ����ѹǿp��ʱ��t�ı仯�����ʾ��
����ϵ����ѹǿp��ʱ��t�ı仯�����ʾ��
t/min | 0 | 5 | 10 | 15 | 20 | �� |
p/kPa | 101.2 | 107.4 | 112.6 | 116.4 | 118.6 | 121.2 |
�������߷�Ӧ�¶���373K����CH3OH(g)�ֽ����ϵѹǿp��(373K)___________121.2kPa(������������������������С����)��ԭ����________________________��
��353Kʱ���÷�Ӧ��ƽ�ⳣ��KP=__________(kPa)2(KPΪ�Է�ѹ��ʾ��ƽ�ⳣ��������������1λС��)��