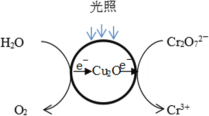
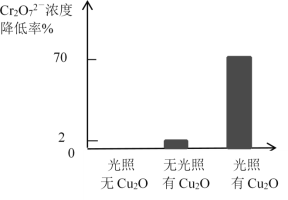
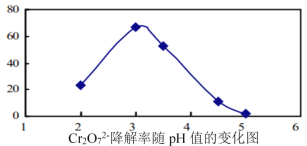
��Ŀ����
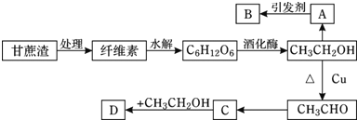
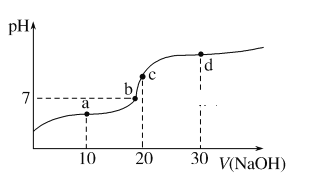
����Ŀ�����ٻ����������ϣ��״�����ķ�Ӧ������ͼ��ʾ�������������ٴ��������ϵ�������*��ע��
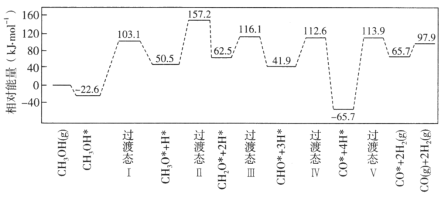
��1��![]() �ġ�H=_____________kJ��mol��1�����������������(���)E��=____________kJ��mol��1��д���ò���Ļ�ѧ����ʽ_________________��
�ġ�H=_____________kJ��mol��1�����������������(���)E��=____________kJ��mol��1��д���ò���Ļ�ѧ����ʽ_________________��
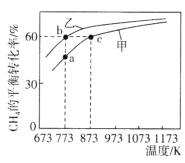
��2����һ���¶��£�CO��H2������巢����Ӧ��![]() ����Ӧ������=����������=k��c(CO)��c2(H2)��k��c(CH3OH)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ������ﵽƽ����������Ч������
����Ӧ������=����������=k��c(CO)��c2(H2)��k��c(CH3OH)��k����k���ֱ�Ϊ�����淴Ӧ���ʳ������ﵽƽ����������Ч������![]() ��___________(����������������С������������)���������¶ȣ�
��___________(����������������С������������)���������¶ȣ�![]() ��____________(����������������С������������)��
��____________(����������������������������)��
��3��353Kʱ���ڸ��������г���CH3OH(g)��������Ӧ��![]() ����ϵ����ѹǿp��ʱ��t�ı仯�����ʾ��
����ϵ����ѹǿp��ʱ��t�ı仯�����ʾ��
t/min | 0 | 5 | 10 | 15 | 20 | �� |
p/kPa | 101.2 | 107.4 | 112.6 | 116.4 | 118.6 | 121.2 |
�������߷�Ӧ�¶���373K����CH3OH(g)�ֽ����ϵѹǿp��(373K)___________121.2kPa(������������������������С����)��ԭ����________________________��
��353Kʱ���÷�Ӧ��ƽ�ⳣ��KP=__________(kPa)2(KPΪ�Է�ѹ��ʾ��ƽ�ⳣ��������������1λС��)��
���𰸡�+97.9 179.6 CO*+4H*=CO*+2H2(g)��4H*=2H2(g) ���� ��С ���� CH3OH(g)CO(g)+2H2(g)������ӦΪ���������������ȷ�Ӧ���¶����ߣ�ƽ�����ƣ��������ʵ������ӣ�����������䣬��ѹǿ��� 43.9
��������
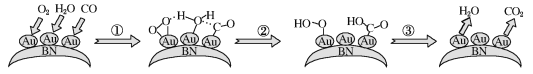
(1)������H=������������-��Ӧ�������������жϣ�����ͼ��Ӧ�����л������ΪCO+4H�Σ�
(2)ƽ��ʱ���淴Ӧ������ȣ������ɼӿ췴Ӧ���ʣ�����Ӱ��ƽ���ƶ�������(1)�з���CH3OH(g)CO(g)+2H2(g)������ӦΪ���ȷ�Ӧ����CO(g)+2H2(g) CH3OH(g)������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���
(3)�ٷ�ӦCH3OH(g)CO(g)+2H2(g)������ӦΪ���������������ȷ�Ӧ��������������Ը�ƽ����ϵ��Ӱ������жϣ�
�ڸ�����ͬ�����£����ʵ���֮�ȵ���ѹǿ֮�ȣ��������ʽ������
(1)����ͼ����ʾ���״����������Ϊ0���������������Ϊ97.9 kJ��mol��1��������H=������������-��Ӧ������������![]() ����H=+97.9kJ��mol��1������ͼ����Կ�����CO+4H= CO+2H2(g)(��4H*=2H2(g))��Ӧ�����л��E�����Ϊ113.9kJ/mol-(-65.7kJ/mol)=179.6 kJ��mol��1��
����H=+97.9kJ��mol��1������ͼ����Կ�����CO+4H= CO+2H2(g)(��4H*=2H2(g))��Ӧ�����л��E�����Ϊ113.9kJ/mol-(-65.7kJ/mol)=179.6 kJ��mol��1��
(2)ƽ��ʱ���淴Ӧ������ȣ�������= ��������![]() =1����Ӧ������=����������=k��c(CO)��c2(H2)��k��c(CH3OH)=0����÷�Ӧ��ƽ�ⳣ��K=
=1����Ӧ������=����������=k��c(CO)��c2(H2)��k��c(CH3OH)=0����÷�Ӧ��ƽ�ⳣ��K=![]() =
=![]() �������ɼӿ췴Ӧ���ʣ�����Ӱ��ƽ���ƶ������淴Ӧ�����������
�������ɼӿ췴Ӧ���ʣ�����Ӱ��ƽ���ƶ������淴Ӧ�����������![]() ���䣻����(1)�з���CH3OH(g)CO(g)+2H2(g)������ӦΪ���ȷ�Ӧ����CO(g)+2H2(g) CH3OH(g)������ӦΪ���ȷ�Ӧ�������¶ȷ�Ӧ�����ƶ���ƽ�ⳣ��K��С����
���䣻����(1)�з���CH3OH(g)CO(g)+2H2(g)������ӦΪ���ȷ�Ӧ����CO(g)+2H2(g) CH3OH(g)������ӦΪ���ȷ�Ӧ�������¶ȷ�Ӧ�����ƶ���ƽ�ⳣ��K��С����![]() ����С��
������
(3)�ٷ�ӦCH3OH(g)CO(g)+2H2(g)������ӦΪ���������������ȷ�Ӧ���¶����ߣ�ƽ�����ƣ��������ʵ������ӣ�����������䣬��ѹǿ��������߷�Ӧ�¶���373K����CH3OH(g)�ֽ����ϵѹǿp��(373K)����121.2kPa��
��353Kʱ����ʼʱƽ����ϵ��ѹǿΪ101.2 kPa��ƽ��ʱ��ϵ��ѹǿΪ121.2kPa�����ʼͶ��CH3OH(g)Ϊ1mol��ƽ��ʱ����CH3OH(g)�����ʵ���Ϊx����������ʽ����
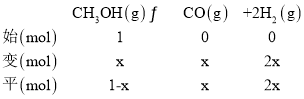
������ͬ�����£����ʵ���֮�ȵ���ѹǿ֮�ȣ���![]() �����x=
�����x=![]() mol����ƽ��ʱ��H2�����ʵ���Ϊ
mol����ƽ��ʱ��H2�����ʵ���Ϊ![]() mol��CH3OH�����ʵ���Ϊ(
mol��CH3OH�����ʵ���Ϊ(![]() )mol��CO�����ʵ���Ϊ
)mol��CO�����ʵ���Ϊ![]() mol��ƽ��ʱ�������ʵ���Ϊ
mol��ƽ��ʱ�������ʵ���Ϊ![]() mol���÷�Ӧ��ƽ�ⳣ��KP
mol���÷�Ӧ��ƽ�ⳣ��KP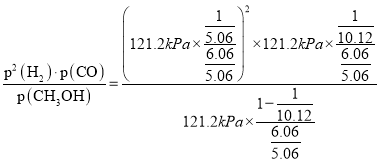 =43.9(kPa)2��
=43.9(kPa)2��