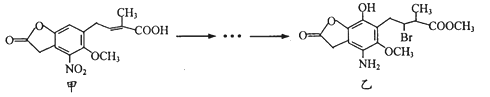
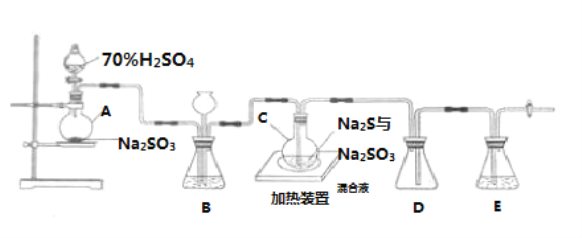
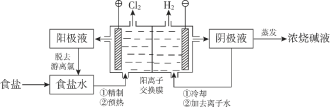
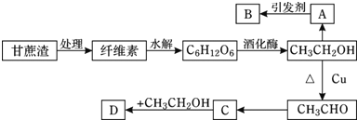
��Ŀ����
����Ŀ�����û�ѧԭ�����ԶԹ����ŷŵķ�ˮ�������Ƚ�����Ч��������������ij�������Ƹ� ������Cr��III���Ĵ��������������¡�
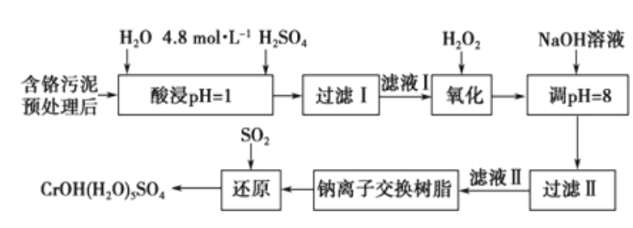
��֪���������ȡ����Һ�еĽ���������Ҫ��Cr3+�������Fe3+��Al3+��Ca2+��Mg2+��
��CrOH��H2O��5SO4������ˮ��
��1��ŨH2O2��Һ�������ǽ���ҺI�е�Cr3+ת��ΪCr2O72-��д���˷�Ӧ�����ӷ���ʽ:____________��
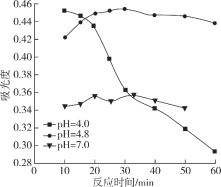
��2�������£�����������������������ʽ����ʱ��Һ��pH���£�
������ | Fe3+ | Mg2+ | Al3+ | Cr3+ |
��ʼ����ʱ��pH | 2.7 | - | - | - |
������ȫʱ��pH | 3.7 | 11.1 | 5.2 | 5.6 |
����NaOH��Һʹ��Һ�ʼ��ԣ�Cr2O72-ת��ΪCr2O42-�������ӷ���ʽΪ______________________���˲�������Һ��pH����8��ͨ��������ȥ�Ľ�������Ϊ___________���˲�����û�м������߽�Mg2+��ȥ��������______________________��
��3�������ӽ�����֬�ķ�Ӧԭ��ΪMn++nNaR��MRn+nNa+�����ø÷�����Ҫ��ȥ����ҺII�е�Mg2+�� �������ҺIIͨ�������ӽ���������Һ��c��Na+��Ϊamol/L������ҺII��c��Mg2+��Ϊ___________��
��4��д��������������SO2���л�ԭʱ������Ӧ�����ӷ���ʽ��_________________________________��
���𰸡�3H2O2+ H2O +2Cr3+==Cr2O72-+ 8H+ Cr2O72-+ 2OH- == 2CrO42-+ H2O Fe3+��Al3+ ��ֹ����̫ǿ��������ת��Ϊƫ��������� a/2 SO2+2CrO42-+12H2O==SO42-+ CrOH��H2O��SO4+ 2OH-
��������
��������Ԥ�������������ȡҺ�н���������Ҫ��Cr3���������Fe3����Al3����Ca2����Mg2�������������������������Ϊ��̬����������������Һ������ҺPH=8ʹ�������ӳ��������˵õ���Һ�������ӽ�����֬����þ���Ӻ����ӣ��õ� ��Һ��ͨ���������ԭ�õ�Cr(OH)(H2O)5SO4��
(1)Cr3��ת��ΪCr2O72����Cr�Ļ��ϼ۴ӣ�3���ߵ���6��H2O2��O�Ļ��ϼ۽��ͣ����ݵ�ʧ�����غ��ԭ���غ㣬д����ѧ����ʽ3H2O2+ H2O +2Cr3+=Cr2O72-+ 8H+��
(2)����OH����Cr2O72��ת��ΪCrO42�����ϼ�û�б仯�����ݵ���غ��ԭ���غ㣬�ɵ����ӷ���ʽCr2O72-+ 2OH- = 2CrO42-+ H2O�����ݱ�������������Ϣ��pH=8ʱ��Fe3����Al3����ȫ��������Һ��Cr����6��Cr����ʽ���ڣ�����û��Cr3���γɵij�����pH=8ʱ����������Fe(OH)3��Al(OH)3��ҪʹMg2��������pH������ߣ�����Al(OH)3�������������Al(OH)3�����ڹ����ļ���Լ��Բ���̫ǿ����ֹ����̫ǿ��������ת��Ϊƫ��������ӣ�
(3)���ݷ�Ӧԭ��ΪMn++nNaR��MRn+nNa+������������ѭ����غ㣬��Mg2�����룬Mg2����2NaR��MR2��2Na����Mg2����Na���ı���Ϊ1��2������֪����������c(Na+)Ϊamol/L����ǰ��c(Mg2��)=a/2mol��L��1��
(4)����������л�ԭ�ԣ�����Һ����ͨ�����ӽ��������Һ��Na2CrO4����Ϊ���ᣬNa2CrO4������ԭΪCrOH(H2O)5SO4��ˮ��Һ����������������Һ�����ᷴӦ���������ƣ�����ԭ���غ������д��ƽ��3SO2+2Na2CrO4+12H2O=2CrOH(H2O)5SO4��+Na2SO4+2NaOH��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�