��Ŀ����
����Ŀ���о���ѧϰС���ͬѧ��Ϊ�ⶨij��ͭ3%��5%����ͭ�Ͻ𣨲�������Ԫ�أ���ͭ����������������������ֲ�ͬʵ�鷽������̽������д���пհס�
[����һ]����ͭ�Ͻ�������NaOH��Һ��Ӧ���ⶨʣ�����������
��1��ʵ���з�����Ӧ�Ļ�ѧ����ʽ��___��
��2����ȡ5.4g��ͭ�Ͻ��ĩ��Ʒ������VmL2.0mol/LNaOH��Һ�С�Ϊʹ�䷴Ӧ��ȫ����NaOH��Һ�����V��___mL�����ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���ͭ������������__���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
[������]����ͭ�Ͻ�������ϡ������Һ��Ӧ������Ͳ���������ܲⶨ����������ͨ��״����Լ20�棬1.01��105Pa���������
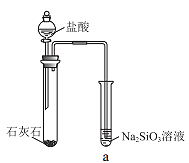
��3��ͬѧ����ѡ��ͼ1ʵ��װ�����ʵ�飺ʵ�鿪ʼʱ���ȴ�Һ©���ϿڵIJ���������������������һ�����ϡ����Ҳ����˳��������ƿ.�����������ԭ��___��
��4��ʵ�����ʱ ������������������Ƶ���___��
��5����ƿ�вд��������ʵ�����Ƿ���Ӱ��___(��С���û�С������жϡ�)
��6��ͬѧ����ϸ����ͼ1ʵ��װ�ú�������Ϊ�������������ϴ���ϡ���������ƿ�У���ʹ������������Ҳ�Ὣƿ�ڿ����ų���ʹ�����������ƫ��ʵ�����ʱ�����ӹ��ƿ����Ͳ�ĵ�����������ˮ���ڣ�ʹ�����������ƫС���������������ͼ2��ʵ��װ�á�
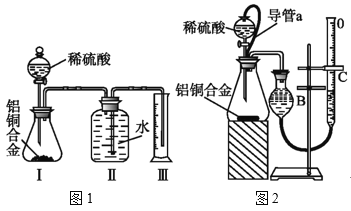
��װ���е���a��������___��
��ʵ����ȷ�ⶨ��ʵ��ǰ��ͭ�Ͻ�����m1g��ʵ�����ͭ�Ͻ�����m2g���������������Ϊ(V2-V1)ml�������������ݼ���ͨ��״���µ�����Ħ�������Vm=___��
���𰸡�2Al+2NaOH+2H2O=2NaAlO2+3H2�� 97mL ƫ�� ����ϡ���ᷴӦ����������ʹ��ƿ����ѹ���� ������Ͳ��ˮ����� û�� ƽ����ƿ���Һ©���ڵ�ѹǿ����֤ϡ����˳�����£������������ڼ���ϡ������������������� ![]() L��mol-1
L��mol-1
��������
����һ����1����������������Һ��Ӧ����ƫ��������������
��2��þ������������Сʱ�������������������Ҫ������������Һ��࣬ʵ����Ҫ����������Һ�����Ӧ���ڻ�������ֵ���ݴ˼��㣻
þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��
��3��þ������ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ���
��4�������������Ƶ��ڽ�����Ͳ��ˮ�������
��5����ƿ�вд��������ʵ����û��Ӱ�죻
��6���ٵ���a�ɱ��ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
�ڸ�����������֮��Ĺ�ϵʽ���㣮
��1����������������Һ��Ӧ����ƫ��������������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2����þΪ3%ʱ���������ĺ�����ߣ�5.4g�Ͻ�����������Ϊ��5.4g����1-3%��=5.4��97%g����
2Al+2NaOH+2H2O=2NaAlO2+3H2��
54g 2mol
5.4g��97%g V��10-3L��2.0mol��L��1
����54g����5.4g��97%g��=2mol����V��10-3L��2.0mol��L��1������ã�V=97����V��NaOH��Һ����97mL��
��2��þ�ϻḽ��ƫ�����Ƶ����ʣ�δϴ�ӵ��²ⶨ��þ������ƫ��þ����������ƫ�ߣ�
����������3��þ������ϡ���ᷴӦ�������������壬ʹ��ƿ������ѹǿ����������˳��������ƿ��
��4�����������ѹǿ����ˮ�Ӽ���ƿ���ų�����������������ˮ�������ռ�����ˮ��������Ƶ��������������ʵ�����ʱ������������������Ƶ��ڽ�����Ͳ��ˮ�������
��5����ƿ�вд��������ʵ����û��Ӱ�죻
��6����װ���е���a�����ã��ܱ��ַ�Һ©��������ѹǿ����ƿ������ѹǿ��ȣ���Һ©������ʱϡ������˳�����£�������ƿ��ϡ����������ڽ����Һ©��������������Ӷ��������ڼ���ϡ������������������
��ʵ����ȷ�ⶨ��ʵ��ǰ��ͭ�Ͻ�����m1g��ʵ�����ͭ�Ͻ�����m2g���������������Ϊ(V2-V1)ml��
2Al+6H��=2Al3��+3H2����
2mol 3mol
![]() mol
mol ![]() mol
mol
����Ħ�������Vm=![]() L��mol-1��
L��mol-1��

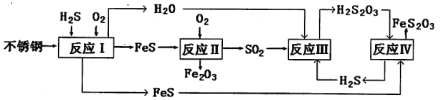
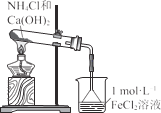
����Ŀ�����������[(NH4)2Fe(SO4)2���Ƿ�����ѧ�е���Ҫ�Լ����ڲ�ͬ�¶��¼��ȷֽ����ò��ﲻͬ�������ͼʵ��װ�ã��г�װ����ȥ������500��ʱ������������A�е�������������ֽ���ȫ��ȷ���ֽ���������������������������ʡ�ˮ���ľ���ɷ֣���֪ÿ��װ���е�ҩƷ��������
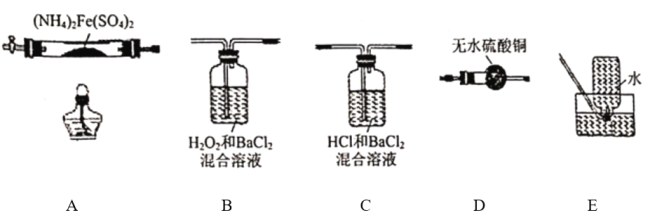
��1����������װ����ɸ�ʵ�飬���װ�õ���ȷ����˳��Ϊ_______________(��װ���������ĸ������˳��ÿ��װ�ö�Ҫʹ�ã�������Aǰ��Ҫͨ��һ��ʱ��N2Ŀ����_______________��
��2�����۲쵽B���а�ɫ�������ɣ�C��������������װ��C������Ϊ_______________,D�а�ɫ��Ϊ��ɫ��д��B�з�����Ӧ�����ӷ���ʽ_______________װ��B��װ��C�ܷ�_______________���� ���ܡ�����ԭ����_______________����ܡ�����˿գ���
��3��A�й�����ȫ�ֽ���Ϊ����ɫ��ĩ��ijͬѧ���ʵ����֤����������ΪFe2O3��������FeO���������ɱ������ݣ��Լ�����������Ʒ��ѡ����
ʵ�鲽�� | Ԥ������ | ���� |
_______________ | _______________ | �̱��������ΪFe2O3 |
��4�������������500��ʱ��������������ȫ�ֽ⣬��E���ռ���������ֻ��N2,A�й��������Fe2O3������Ϊ80g,B�г������ʵ���Ϊ2rnol,������N2������Ϊ_______________g��
��5��ij����(Cr2O72-����ˮ�������������Һ�ζ���������Ӧ����Ԫ�غ�Ԫ����ȫת��Ϊ�����������и�Ԫ�ػ��ϼ�Ϊ+3),�ó����������õ�amolFeOFemCrnO3�������Ǵ��������е�ʵ����ģ���������������淋����ʵ���Ϊ_______________mol(��a�Ĵ���ʽ��ʾ����
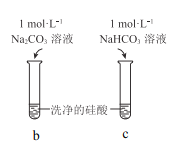
����Ŀ�����ᣨH2SiO3����һ��������ˮ�����ᣬ����Һ������ʱ���γ�����״������ʵ���ҳ���Na2SiO3��Һ�Ʊ����ᡣijС��ͬѧ����������ʵ�飺���н��۲���ȷ���ǣ� ��
��� | �� | �� |
ʵ�� |
|
|
���� | a �в�������״���� | b ������״�����ܽ⣬c �������Ա仯 |
A.Na2SiO3��Һһ���Լ���
B.�ɢ���˵������H2CO3��H2SiO3
C.�ɢ��֪��ͬŨ��ʱNa2CO3��Һ�ļ���ǿ��NaHCO3��Һ
D.��Na2SiO3��Һ��ͨ������CO2��������Ӧ��SiO32��+CO2+H2O=CO32��+H2SiO3��
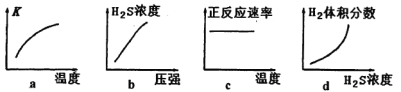
����Ŀ������ʵ������Ԥ����ȷ����
A | B | C | D |
|
|
|
|
�ձ��в�����ɫ������һ��ʱ�����������Ա仯 | ��������ְ�ɫ���ǣ����ȱ���� | KMnO4 ������Һ�ڱ��ͼױ��о���ɫ | Һ��ֲ㣬�²�� ��ɫ |
A.AB.BC.CD.D