��Ŀ����
����Ŀ�������Ԫ�آ٣����ڱ��е�λ�ã������жϳ���Ԫ�ػش����⣺
�� | |||||||
�� | �� | �� | |||||
�� | �� | �� | �� |
��1����ԭ�ӵ��������ӵ��������ʽΪ___________________,�������������p�Dz���ӵ�˵���������_________��������ţ�
a. ������ͬ b.��������״��ͬ
c. ����������ͬ d. ��������չ������ͬ
��2���ۺ͢���ɵ��ĺ˷��ӵĻ�ѧʽΪ___________���ռ乹��Ϊ_____________������_____���ӣ�����ԡ��Ǽ��ԡ�����
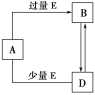
��3���ȽϢܡ���Ԫ�س��������İ뾶��С���û�ѧʽ��ʾ��______��_____���ڡ��� ��Ԫ�طǽ����Խ�ǿ���� ______��дԪ�ط��ţ���д��֤���ý��۵�һ����ʵ:___________________________ ��
��4���ܡ���Ԫ���γɵ���ԭ�ӻ����������ʽΪ ___________ ����֪��Ԫ�ص���������۵�Ϊ2054�棬������Ϊԭ�ϣ�����ͨ�����ķ����õ������ݴ��ƶϢ�Ԫ�ص�������Ϊ_________���壨����Ӿ��塱�����Ӿ��塱��ԭ�Ӿ��塱�������塱������Ԫ�ص��������۵�______��Ԫ�ص��Ȼ����۵㣨����ڡ���С�ڡ������ڡ������������ԭ��______________________________��
���𰸡�![]() cd NH3 ������ ���� O2- Na+ N ��ۺ���������ԣ����̼��
cd NH3 ������ ���� O2- Na+ N ��ۺ���������ԣ����̼�� ![]() ���� ���� ������Ϊ���Ӿ��壬�Ȼ���Ϊ���Ӿ��壬һ�����Ӿ�����۷е���ڷ��Ӿ�����۷е�
���� ���� ������Ϊ���Ӿ��壬�Ȼ���Ϊ���Ӿ��壬һ�����Ӿ�����۷е���ڷ��Ӿ�����۷е�
��������
����Ԫ�������ڱ��е�λ�ÿ�֪������H������C������N������O������Na������Al������S������Cl�����ԭ�ӽṹ�����ʷ������
����Ԫ�������ڱ��е�λ�ÿ�֪������H������C������N������O������Na������Al������S������Cl��
(1)����Cl��ԭ�ӵ�����������7�����ӣ��������ʽΪ![]() �� a. �����p�Dz������ͬһ�ܼ��ϣ�������ͬ����a��ȷ��b. p�Dz����Ϊ�Ĵ��Σ���������״��ͬ����b��ȷ��c. ͬһ����ĵ������������෴����c����d. p�Dz���3��p�������������չ����ͬ�����ֱ����d���ʴ�Ϊ��
�� a. �����p�Dz������ͬһ�ܼ��ϣ�������ͬ����a��ȷ��b. p�Dz����Ϊ�Ĵ��Σ���������״��ͬ����b��ȷ��c. ͬһ����ĵ������������෴����c����d. p�Dz���3��p�������������չ����ͬ�����ֱ����d���ʴ�Ϊ��![]() ��cd��
��cd��
(2)�ۺ͢���ɵ��ĺ˷���Ϊ��������ѧʽΪNH3��NH3��Nԭ�ӳ�3����������һ��δ�ɼ��ŶԵ��ӣ��ӻ������Ϊ4����ȡsp3���ӻ����������ӿռ乹���������Σ�������������ɵ����IJ��غϣ����ڼ��Է��ӣ��ʴ�Ϊ��NH3�������Σ����ԣ�
(3)����O������Na�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��ԭ������Խ�����Ӱ뾶ԽС�����Ӱ뾶O2-��Na+������C������N����Ԫ���зǽ����Խ�ǿ���� N����Ϊ������ǿ�ᣬ��̼��Ϊ���ᣬ����ۺ���������ԣ����̼�ᣬ�ʴ�Ϊ��O2-��Na+��N����ۺ���������ԣ����̼�
(4)����O������Na���ܡ���Ԫ���γɵ���ԭ�ӻ�����Ϊ�������ƣ��������ӻ��������ʽΪ![]() ����֪���������۵�Ϊ2054����������Ϊԭ�ϣ�����ͨ�����ķ����õ���������������������Ӿ��塣������Ϊ���Ӿ��壬�Ȼ���Ϊ���Ӿ��壬������������۵�����Ȼ������۵㣬һ�����Ӿ�����۷е���ڷ��Ӿ�����۷е㣬�ʴ�Ϊ��
����֪���������۵�Ϊ2054����������Ϊԭ�ϣ�����ͨ�����ķ����õ���������������������Ӿ��塣������Ϊ���Ӿ��壬�Ȼ���Ϊ���Ӿ��壬������������۵�����Ȼ������۵㣬һ�����Ӿ�����۷е���ڷ��Ӿ�����۷е㣬�ʴ�Ϊ��![]() �����ӣ�����������Ϊ���Ӿ��壬�Ȼ���Ϊ���Ӿ��壬һ�����Ӿ�����۷е���ڷ��Ӿ�����۷е㡣
�����ӣ�����������Ϊ���Ӿ��壬�Ȼ���Ϊ���Ӿ��壬һ�����Ӿ�����۷е���ڷ��Ӿ�����۷е㡣

����Ŀ��ȼú�������к��� SO2��Ϊ�����������������������ö��ַ���ʵ����������
��.(1)��ʪʽ���շ����������ռ��� SO2 ������Ӧ�Ӷ����������Լ����ʺ������÷����ռ�����_____(����ĸ���)��
a. ʯ���� b.CaCl2��Һ
(2)ij�������ú� SO2 ������������Cr2O72-�����Է�ˮ���������з�Ӧ��ĸ�Ԫ����Cr3+��ʽ���ڣ������������£�

���� SO2 ����������ˮʱ�������� SO2 ��_____�ԡ�
���������з�����Ӧ�����ӷ���ʽΪ_____��
��.ʯ��-ʯ�෨���ռ�dz��õ���������ʯ��-ʯ�෨�����շ�ӦΪCa(OH)2+SO2= CaSO3��+H2O�����ղ�����������ɹܵ���������������������ӦΪ2CaSO3+O2+4H2O =2CaSO4��2H2O����������ͼ��

�ռ�����շ�ӦΪ2NaOH+SO2=Na2SO3+H2O���÷����ص����������Ƽ���ǿ�����տ졢Ч�ʸߡ���������ͼ��

��֪��
�Լ� | Ca(OH)2 | NaOH |
�۸�(Ԫ/kg) | 0.36 | 2.9 |
���� SO2 �ijɱ�(Ԫ/mol) | 0.027 | 0.232 |
(3)ʯ��-ʯ�෨���ռ��ȣ�ʯ��-ʯ�෨���ŵ���_______��ȱ����_______��
(4)ijѧϰС����ʯ��-ʯ�෨���ռ�Ļ����ϣ����һ���Ľ��ġ���ʵ������ѭ������������������ͼ�еļס��ҡ�������_____��_____��_____(�ѧʽ)