��Ŀ����
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)����������£�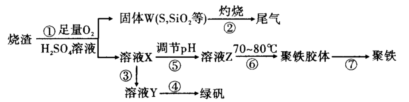
(1)���̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(2)���̢��в�����β����Դ��������Ⱦ����ѡ�������Լ��е� ���ա�
a��ŨH2SO4 b������ˮ c��NaOH��Һ d��Ũ����
(3)���̢��У���Ҫ���������������
(4)���̢ܵ�ʵ�������
(5)���̢��У�����ҺZ���ȵ�70��80�棬Ŀ���� ��
(6)ʵ����Ϊ�ⶨ���õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ��Ʒ2.700 g���ڽ���Ʒ����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495 g�����þ�����Ҫ�ɷ�Ϊ[Fe(OH)SO4]n����þ�������Ԫ�ص���������Ϊ ��
��1��4FeS+3O2+6H2SO4=2Fe2(SO4)3+6H2O+4S��2�֣�
��2��c��2�֣� ��3������2�֣�
��4��������Ũ����������ȴ���ᾧ�����ˡ�ϴ�ӣ�4�֣�Ũ������ȴ��д���Բ��۷֣�
��5���ٽ�Fe3+��ˮ�⣨2�֣�
��6��31.11%��3�֣�
�������������
��1�����ݹ���W�ijɷ��д���S���Ƴ�FeS��O2��H2SO4����������ԭ��Ӧ��FeS����ԭ����O2����������������Fe2(SO4)3��S��H2O��
��2����Ⱦ����SO2���ü�Һ���ա�
��3����ҺX�е�����Fe3+���̷��е���ΪFe2+����Ӧ����Fe�ۣ���Fe3+��ԭΪFe2+��
��4�������γɽᾧˮ���������Һ���ܲ�ȡֱ�����ɵİ취��ȡ���塣
��5�������¶ȣ��ٽ�Fe3+��ˮ�⡣ ��6���۵õ��ij�����BaSO4��n(BaSO4) =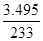 =0.015mol�����ݾ�����Ҫ�ɷ�Ϊ[Fe(OH)SO4] n���Ƴ�n(Fe3+)=0.015mol����m(Fe3+)=0.84g����Ԫ�ص���������:��(Fe)=(0.84g/2.700g)��100%=31.11%��
=0.015mol�����ݾ�����Ҫ�ɷ�Ϊ[Fe(OH)SO4] n���Ƴ�n(Fe3+)=0.015mol����m(Fe3+)=0.84g����Ԫ�ص���������:��(Fe)=(0.84g/2.700g)��100%=31.11%��
���㣺�Թ�������Ϊ���壬������Ԫ�ؼ��������ѧʵ�顢��ѧ���㡢������ԭ��Ӧ��֪ʶ��

С����ϲ����ѧʵ��Σ�����Ҫѧϰ��̽�������仯����������Ի�ԭ�ԡ���������һ���߽����Ļ�ѧ���á�
��1����ǰ��ʦ����������Ԥϰ��ҵ������һ����ɣ�
������ͬ��̬�����ʸ�дһ��(�������Ԫ�صĻ��ϼ�)��_____��_______�� ________��
��д��һ������֮���ת��(�����ּ�̬)�Ļ�ѧ����ʽ��___________________ ��
��2��ʵ�����ṩ�������Լ���п�������ۡ�0.1 mol��L��1 FeCl3��Һ��0.1 mol��L��1 FeCl2��Һ��KSCN��Һ��������ˮ��̽��Fe2����Fe3���������ԡ���ԭ�ԡ�
����������ԭ��Ӧ���й�ԭ����С��˵Fe2�����л�ԭ�����������ԣ�Ϊ֤ʵ�Լ��ļ��裬�����С��һ�����ʵ�鷽��������ʵ�鲢����ʵ������������б���
| ̽������ | ʵ�鷽�� | ʵ������ |
| ̽��Fe2�����л�ԭ�� | ȡ����0.1 mol��L��1 FeCl2��Һ����������__________��������Һ�м�������__________ | ��Һ���Ѫ��ɫ |
| ̽��Fe2������������ | ȡ����0.1 mol��L��1 FeCl2�� Һ������_________��� ��Ӧ | ��Һ��dz��ɫ����ɫ ��������Ӧ���ӷ���ʽΪ________________ [��Դ:Z|xx|k.Com] |
�̷���FeSO4��7H2O��������ȱ����ƶѪҩƷ����Ҫ�ɷ֡���������������м�����������������������ʣ�Ϊԭ�����������̷���һ�ַ�����
��ѯ���ϣ����й����ʵ��������±���
| 25��ʱ | pHֵ |
| ����H2S��Һ | 3.9 |
| SnS������ȫ | 1.6 |
| FeS��ʼ���� | 3.0 |
| FeS������ȫ | 5.5 |
��1�������Ƶõ��̷��������Ƿ���Fe3+�����ѡ�õ��Լ�Ϊ ��
A��KSCN��Һ B��NaOH��Һ C��KMnO4��Һ D��������Һ
��2������II�У�ͨ�����������͵�Ŀ���� ������Һ���������ữ��pH=2��Ŀ���� ��
��3������IV��˳������Ϊ ����ȴ�ᾧ�� ��
��4������IV�õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ��ٳ�ȥ������渽�ŵ���������ʣ��� ��
��5���ⶨ�̷���Ʒ��Fe2+�����ķ����ǣ�a.��ȡ2.850g�̷���Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�b.��ȡ25.00mL������Һ����ƿ�У�c.�������ữ��0.01000mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
�ٵζ�ʱʢ��KMnO4��Һ������Ϊ �����������ƣ���
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����� ��
�ۼ���������Ʒ��FeSO4��7H2O����������Ϊ ��
��ͼ����ע�����м�������Na2SO3���壬����������Ũ����(�Բ��Ӵ�ֽ��Ϊ)���������й�˵����ȷ����(����) 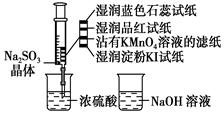
| A����ɫʯ����ֽ�ȱ�����ɫ |
| B��Ʒ����ֽ��մ��KMnO4��Һ��ֽ����ɫ����֤��SO2��Ư���� |
| C��ʪ�����KI��ֽδ����˵��SO2������������I2 |
| D��NaOH��Һ��Ʒ����Һ�������ڳ�ȥʵ���ж����SO2 |
������Һ���ܹ�����SO2��CO2�������
�ٳ���ʯ��ˮ ��KMnO4������Һ ����ˮ ��Ʒ����Һ
| A���٢ۢ� | B���ڢۢ� | C���٢ڢ� | D��ȫ�� |
 Fe��SCN��3���з�ӦѸ�١��������Ե��ص㣬�Ǽ���Fe3�����õķ���֮һ��ij��ѧ��ȤС��Ϊ̽��Fe��SCN��3�����ʣ���������ʵ�飺
Fe��SCN��3���з�ӦѸ�١��������Ե��ص㣬�Ǽ���Fe3�����õķ���֮һ��ij��ѧ��ȤС��Ϊ̽��Fe��SCN��3�����ʣ���������ʵ�飺
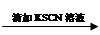 ����������
����������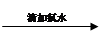 ��Һ���
��Һ��� ��Һ��ɫδ��ȥ
��Һ��ɫδ��ȥ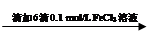 ��Һ���Ա��
��Һ���Ա�� 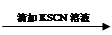 ��Һ���
��Һ��� ��Һ����
��Һ���� ȡ�ϲ���Һ
ȡ�ϲ���Һ ��Һ���������Թ�2����Һ��ɫ�
��Һ���������Թ�2����Һ��ɫ�
 2NaAlO2��aq��+4H2O��[Al2O3?3H2OҲ�ɱ�ʾΪ2 Al(OH)3]
2NaAlO2��aq��+4H2O��[Al2O3?3H2OҲ�ɱ�ʾΪ2 Al(OH)3]