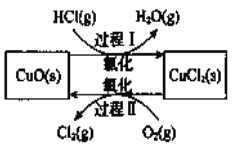
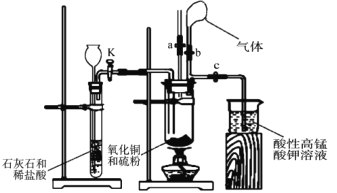
ĚâÄżÄÚČÝ
ˇľĚâÄżˇżTˇćʱŁ¬ÔÚŇ»¸öĚĺ»ýÎŞ2LµÄČÝĆ÷ÖĐŁ¬AĆřĚĺÓëBĆřĚĺ·´Ó¦ÉúłÉCĆřĚ壬·´Ó¦ąýłĚÖĐAˇ˘Bˇ˘C±ä»ŻČçÍĽËůĘľˇŁ
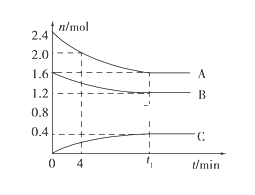
Ł¨1Ł©Đ´łö¸Ă·´Ó¦µÄ»ŻŃ§·˝łĚĘ˝Łş___________Ł»
Ł¨2Ł©¸ĂζČϸ÷´Ó¦µÄĆ˝şâłŁĘýÎŞŁ¨±ŁÁôÁ˝Î»ÓĐЧĘý×ÖŁ©_______Ł»
Ł¨3Ł©ŇŃÖŞŁşKŁ¨300ˇćŁ©>KŁ¨350ˇćŁ©Ł¬¸Ă·´Ó¦ĘÇ________ČČ·´Ó¦Ł»
Ł¨4Ł©0ˇ«4·ÖÖÓʱŁ¬AµÄĆ˝ľů·´Ó¦ËŮÂĘÎŞ____________Ł»
Ł¨5Ł©µ˝´ďĆ˝şâʱBµÄת»ŻÂĘÎŞ____________Ł»
Ł¨6Ł©şăČÝĚőĽţĎÂŁ¬ĎÂÁĐ´ëĘ©ÖĐÄÜĘą![]() ˝µµÍµÄÓĐ__________ˇŁ
˝µµÍµÄÓĐ__________ˇŁ
A łäČ뺤Ćř B ĘąÓĂ´ß»ŻĽÁ
C ÔŮłäČë2.4mol AşÍ1.6mol B D ˝µµÍζČ
ˇľ´đ°¸ˇż2AŁ¨gŁ©Ł«BŁ¨gŁ©![]() CŁ¨gŁ© 0.52 ·Ĺ 0.05molˇ¤LŁ1ˇ¤minŁ1 25% Cˇ˘D
CŁ¨gŁ© 0.52 ·Ĺ 0.05molˇ¤LŁ1ˇ¤minŁ1 25% Cˇ˘D
ˇľ˝âÎöˇż
Ł¨1Ł©¸ůľÝÍĽĎóÖŞŁ¬Aˇ˘BĘÇ·´Ó¦ÎCĘÇÉúłÉÎƽşâʱˇ÷n(A)=(2.4-1.6)mol=0.8molˇ˘ˇ÷n(B)=(1.6-1.2)mol=0.4molˇ˘ˇ÷n(C)=(0.4-0)mol=0.4molŁ¬Í¬Ň»żÉÄć·´Ó¦ÖС˘Í¬Ň»Ę±Ľä¶ÎÄÚ¸÷ÎďÖʵÄÎďÖʵÄÁż±ä»ŻÁżÖ®±ČµČÓÚĆäĽĆÁżĘýÖ®±ČŁ¬ľÝ´ËĘéĐ´Ćä·˝łĚĘ˝Ł»
Ł¨2Ł©¸ĂζČϸ÷´Ó¦µÄĆ˝şâłŁĘýµČÓÚÉúłÉÎďŨ¶ČĽĆÁżĘýĂÝÖ®»ýÓë·´Ó¦ÎďŨ¶ČĽĆÁżĘýĂÝÖ®»ýµÄ±ČŁ»
Ł¨3Ł©ÉýÎÂKµÄĽőСŁ¬ËµĂ÷Éý¸ßζČĆ˝şâÄćĎňŇƶŻŁ»
Ł¨4Ł©0ˇ«4·ÖÖÓʱŁ¬CµÄĆ˝ľů·´Ó¦ËŮÂĘÎŞ=![]() Ł»
Ł»
Ł¨5Ł©µ˝´ďĆ˝şâʱBµÄת»ŻÂĘ=![]() Ł»
Ł»
Ł¨6Ł©şăČÝĚőĽţĎÂŁ¬ÄÜĘą![]() ĽőСŁ¬żÉÄÜĘÇAµÄÎďÖʵÄÁżĽőС»ňCµÄÎďÖʵÄÁżÔöĽÓŁ»
ĽőСŁ¬żÉÄÜĘÇAµÄÎďÖʵÄÁżĽőС»ňCµÄÎďÖʵÄÁżÔöĽÓŁ»
Ł¨1Ł©¸ůľÝÍĽĎóÖŞŁ¬Aˇ˘BĘÇ·´Ó¦ÎCĘÇÉúłÉÎƽşâʱˇ÷n(A)=(2.4-1.6)mol=0.8molˇ˘ˇ÷n(B)=(1.6-1.2)mol=0.4molˇ˘ˇ÷n(C)=(0.4-0)mol=0.4molŁ¬Í¬Ň»żÉÄć·´Ó¦ÖС˘Í¬Ň»Ę±Ľä¶ÎÄÚ¸÷ÎďÖʵÄÎďÖʵÄÁż±ä»ŻÁżÖ®±ČµČÓÚĆäĽĆÁżĘýÖ®±Č=0.8molŁş0.4molŁş0.4mol=2Łş1Łş1Ł¬Ôň¸Ă·´Ó¦·˝łĚʽΪ2A(g)+B(g)![]() C(g)Ł»
C(g)Ł»
Ł¨2Ł©Ć˝şâʱcŁ¨AŁ©=![]() ˇ˘cŁ¨BŁ©=
ˇ˘cŁ¨BŁ©=![]() ˇ˘cŁ¨CŁ©=
ˇ˘cŁ¨CŁ©=![]() Ł¬¸ĂζČϸ÷´Ó¦µÄĆ˝şâłŁĘý=
Ł¬¸ĂζČϸ÷´Ó¦µÄĆ˝şâłŁĘý=![]() 0.52Ł»
0.52Ł»
Ł¨3Ł©ÉýÎÂKĽőСŁ¬ËµĂ÷Éý¸ßζČĆ˝şâÄćĎňŇƶŻŁ¬Őý·´Ó¦·ĹČČŁ»
Ł¨4Ł©0ˇ«4·ÖÖÓʱŁ¬CµÄĆ˝ľů·´Ó¦ËŮÂĘÎŞ=![]() =0.05molˇ¤LŁ1ˇ¤minŁ1Ł»
=0.05molˇ¤LŁ1ˇ¤minŁ1Ł»
Ł¨5Ł©µ˝´ďĆ˝şâʱBµÄת»ŻÂĘ=![]() =25% Ł»
=25% Ł»
Ł¨6Ł©AŁ®łäČ뺤ĆřŁ¬·´Ó¦ÎPÉúłÉÎďŨ¶Č˛»±äŁ¬ÔňĆ˝şâ˛»ŇƶŻŁ¬ËůŇÔ![]() ˛»±äŁ¬ąĘA´íÎóŁ»
˛»±äŁ¬ąĘA´íÎóŁ»
BŁ®ĘąÓĂ´ß»ŻĽÁŁ¬Äܸı䷴ӦËŮÂĘËő¶Ě·´Ó¦´ďµ˝Ć˝şâµÄʱĽäŁ¬Ć˝şâ˛»ŇƶŻŁ¬ËůŇÔ![]() ˛»±äŁ¬ąĘB´íÎóŁ»
˛»±äŁ¬ąĘB´íÎóŁ»
CŁ®ÔŮłäČë2.4molAşÍ1.6molBŁ¬Ďŕµ±ÓÚÔö´óѹǿŁ¬Ć˝şâĎňŐý·´Ó¦·˝ĎňŇƶŻŁ¬ËůŇÔ![]() ĽőСŁ¬ąĘCŐýČ·Ł»
ĽőСŁ¬ąĘCŐýČ·Ł»
DŁ®˝µµÍζȣ¬Ć˝şâĎňŐý·´Ó¦·˝ĎňŇƶŻŁ¬Ôň![]() ĽőСŁ¬ąĘDŐýČ·Ł»ŃˇCDˇŁ
ĽőСŁ¬ąĘDŐýČ·Ł»ŃˇCDˇŁ

 ѧÁ·żěłµµŔżěŔÖĽŮĆÚş®ĽŮ×÷ҵϵÁĐ´đ°¸
ѧÁ·żěłµµŔżěŔÖĽŮĆÚş®ĽŮ×÷ҵϵÁд𰸡ľĚâÄżˇżÇâĆřĘÇÖŘŇŞµÄÇĺ˝ŕÄÜÔ´ˇŁżĆѧĽŇł˘ĘÔ¶ŕÖÖ·˝·¨ÖĆȡÇâĆřˇŁ
IŁ®(1)´˘Çâ˛ÄÁĎ![]() ÄÜÓëË®·´Ó¦µĂµ˝ÇâĆřˇŁÇëĐ´łö
ÄÜÓëË®·´Ó¦µĂµ˝ÇâĆřˇŁÇëĐ´łö![]() µÄµç×ÓĘ˝______________Ł¬¸Ă·´Ó¦µÄ»ŻŃ§·˝łĚʽΪ______________________________ˇŁ
µÄµç×ÓĘ˝______________Ł¬¸Ă·´Ó¦µÄ»ŻŃ§·˝łĚʽΪ______________________________ˇŁ
˘ňŁ®¸ĘÓÍşÍË®ŐôĆřˇ˘ŃőĆřľ´ß»ŻÖŘŐű»ň˛ż·Ö´ß»ŻŃő»ŻżÉÖƵĂÇâĆřŁ¬·´Ó¦Ö÷ŇŞąýłĚČçĎÂŁş
¸ĘÓÍË®ŐôĆřÖŘŐű( |
|
¸ĘÓͲż·ÖŃő»Ż( |
|
¸ĘÓÍŃő»ŻË®ŐôĆűÖŘŐű( |
|
(2)·´Ó¦![]() µÄH1=_____ kJmol-1ˇŁ
µÄH1=_____ kJmol-1ˇŁ
(3)ʵĽĘÉú˛úÖĐ˝«·´Ó¦![]() É趨ÔÚ600ˇ«700ˇć˝řĐĐŁ¬ŃˇÔń¸Ăζȷ¶Î§µÄÔŇňĘÇŁş________________ˇŁ
É趨ÔÚ600ˇ«700ˇć˝řĐĐŁ¬ŃˇÔń¸Ăζȷ¶Î§µÄÔŇňĘÇŁş________________ˇŁ
(4)·´Ó¦![]() µÄ¸±˛úÎďşÜ¶ŕŁ¬ĽÓČëŇ»¶¨ÁżµÄ
µÄ¸±˛úÎďşÜ¶ŕŁ¬ĽÓČëŇ»¶¨ÁżµÄ![]() ͨČëĘʵ±ąýÁżµÄ
ͨČëĘʵ±ąýÁżµÄ![]() ¶ĽÄÜĚá¸ßÇâĆřµÄ˛úÂʡŁÔňĽÓČë
¶ĽÄÜĚá¸ßÇâĆřµÄ˛úÂʡŁÔňĽÓČë![]() µÄÔŇňŁş___________________Ł»Čô»ěşĎĆřĚĺÖĐŁ¬
µÄÔŇňŁş___________________Ł»Čô»ěşĎĆřĚĺÖĐŁ¬![]() ±ČŔýąý¸ßŁ¬Ôň
±ČŔýąý¸ßŁ¬Ôň![]() ˛úÂĘ˝µµÍŁ¬ĆäÔŇňĘÇŁş____________________________ˇŁ
˛úÂĘ˝µµÍŁ¬ĆäÔŇňĘÇŁş____________________________ˇŁ
(5)ͨłŁ˝«![]() ·ÖɢÔڸ߱ȱíĂćµÄÔŘĚĺ(
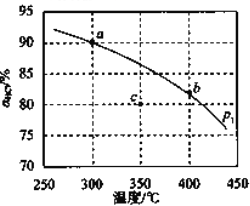
·ÖɢÔڸ߱ȱíĂćµÄÔŘĚĺ(![]() )ÉĎŇÔĚá¸ß´ß»ŻĐ§ÂʡŁ·Ö±đÓĂČýÖÖ´ß»ŻĽÁµÄÔŘĚĺ˝řĐĐʵŃ飬łÖĐřͨČëÔÁĎĆřŇ»¶ÎʱĽäŁ¬»ćÖĆÓÍת»ŻÂĘÓëʱĽäµÄąŘϵČçÍĽËůĘľˇŁ
)ÉĎŇÔĚá¸ß´ß»ŻĐ§ÂʡŁ·Ö±đÓĂČýÖÖ´ß»ŻĽÁµÄÔŘĚĺ˝řĐĐʵŃ飬łÖĐřͨČëÔÁĎĆřŇ»¶ÎʱĽäŁ¬»ćÖĆÓÍת»ŻÂĘÓëʱĽäµÄąŘϵČçÍĽËůĘľˇŁ

˘Ů˝áşĎÉĎÍĽ·ÖÎö![]() ´ß»ŻĽÁľßÓеÄÓŵăĘÇ____________________ˇŁ
´ß»ŻĽÁľßÓеÄÓŵăĘÇ____________________ˇŁ
˘ÚŃĐľż·˘ĎÖÔěłÉ´ß»ŻĐ§ÂĘËćʱĽäĎ½µµÄÖ÷ŇŞÔŇňĘǸ±·´Ó¦˛úÉúµÄ´óÁżĚĽ·Ű(»ýĚĽ)°üąü´ß»ŻĽÁŁ¬Í¨ąýĽÓČë΢ÁżµÄˇ˘żÉŃ»·ŔűÓõÄŃő»Żďç(![]() )żÉÓĐЧĽőÉŮ»ýĚĽˇŁĆä·´Ó¦»úŔí°üŔ¨Á˝˛˝Łş
)żÉÓĐЧĽőÉŮ»ýĚĽˇŁĆä·´Ó¦»úŔí°üŔ¨Á˝˛˝Łş
µÚŇ»˛˝ÎŞŁş![]()
µÚ¶ţ˛˝ÎŞŁş___________________________(Đ´łö»ŻŃ§·´Ó¦·˝łĚĘ˝)ˇŁ