��Ŀ����
��֪����1 mol C��H����Ҫ��������414.4 kJ������1 mol C��C����Ҫ��������347.4 kJ������1 mol C===C������ų�����615.3 kJ������1 mol H��H������ų�����435.3 kJ��ij�л���ֽ�ķ�Ӧ�ɱ�ʾΪ��
���ڷ�Ӧ��������1 mol����(��Ӧ��)�����йظ÷�Ӧ��˵����ȷ����(����)
| A���÷�Ӧ�ų�251.2 kJ������ | B���÷�Ӧ����251.2 kJ������ |
| C���÷�Ӧ�ų�125.6 kJ������ | D���÷�Ӧ����125.6 kJ������ |
D
�������������1mol���麬��1molC-C����6molC-H�������ɵ���ϩ��1molC=C��1molH-H����4molC-H������H=347.4KJ+6��414.4KJ-4��414.4KJ-615.3KJ-435.3KJ=+125.6KJ�����Ը÷�Ӧ����125.6KJ����������D����ȷ��
���㣺���⿼�黯ѧ��Ӧ�������仯��

���и����У����ɡ����ۼ������ϵ����ȷ����
| ѡ�� | ���� | ���� |
| A | ���ܣ�N��N��Cl��Cl | ���ʷе㣺N2��Cl2 |
| B | �����пɵ����H+������H2SO4��CH3COOH | ���ԣ�H2SO4��CH3COOH |
| C | Ԫ�صķǽ����ԣ�N��P | ���ԣ�HNO3��H3PO4 |
| D | �����ԣ�Fe3+��Cu2+ | ��ԭ�ԣ�Fe2+��Cu |
����˵����ȷ���ǣ� ��
| A�������Է����еķ�Ӧ�������ȷ�Ӧ |
| B����ѧ������ʱ���յ��������ڻ�ѧ���γ�ʱ�ų��������ķ�Ӧ���ڷ��ȷ�Ӧ |
| C�����߷�Ӧ���¶ȣ�����Ӱٷ������ӣ����Ӽ���Ч��ײ�ļ�����ߣ���Ӧ�������� |
| D������ͨ���ı䷴Ӧ·����ʹ��Ӧ�����������ı䷴Ӧ����Ļ�� |
��֪����1 mol C��H����Ҫ��������414.4 kJ������1 mol C��C����Ҫ��������347.4 kJ������1 mol C===C������ų�����615.3 kJ������1 mol H��H������ų�����435.3 kJ��ij�л���ֽ�ķ�Ӧ�ɱ�ʾΪ��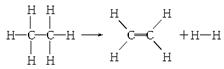
���ڷ�Ӧ��������1 mol���飬���йظ÷�Ӧ��˵����ȷ����(����)
| A���÷�Ӧ�ų�251.2 kJ������ | B���÷�Ӧ����251.2 kJ������ |
| C���÷�Ӧ�ų�125.6 kJ������ | D���÷�Ӧ����125.6 kJ������ |
���й�����Դ����Ϊ��Դ�����ʵ������������
| A�����ȷ�Ӧ�ķ�Ӧ������������������������ |
| B����ɫֲ����й������ʱ��������ת��Ϊ��ѧ�ܡ����桱���� |
| C�����ʵĻ�ѧ�ܿ����ڲ�ͬ������תΪ���ܡ����ܡ����ܵ�Ϊ���������� |
| D����Ȼ�����ڶ�����Դ |
��֪��
(1)����ʧˮ���Ȼ�ѧ����ʽΪCuSO4��5H2O(s)===CuSO4(s)��5H2O(l) ��H����Q1 kJ/mol
(2)�����£���ˮ����ͭ����ˮ���Ȼ�ѧ����ʽΪCuSO4(s)===Cu2��(aq)��SO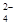 (aq) ��H��
(aq) ��H��
��Q2 kJ/mol
(3)����(CuSO4��5H2O)����ˮʱ��Һ�¶Ƚ��͡���Q1��Q2�Ĺ�ϵ��(Q1��Q2Ϊ����) �� ��
| A��Q1>Q2 | B��Q1��Q2 | C��Q1<Q2 | D����ȷ�� |
CO(g)+H2O(g)  H2(g)+CO2(g) ��H��0���������������������£�����˵����ȷ����
H2(g)+CO2(g) ��H��0���������������������£�����˵����ȷ����
| A������������ı��˷�Ӧ��;������Ӧ�ų�������Ҳ��֮�ı� |
| B���ı�ѹǿ��ƽ�ⲻ�����ƶ�����Ӧ�ų����������� |
| C�������¶ȣ���Ӧ���ʼӿ죬��Ӧ�ų����������� |
| D������ԭ����н��У���Ӧ�ų����������� |
����ɻ��õ�������������(NH4ClO4)�Ļ����Ϊ����ȼ�ϣ���ȼʱ����������������������立�Ӧ���䷽��ʽ�ɱ�ʾΪ��2NH4ClO4  N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� ��
N2��+ 4H2O+Cl2��+2O2�����ų�����ΪQ�����жԴ˷�Ӧ�����д�����ǣ� ��
| A����Ӧ���ڷֽⷴӦ |
| B��������Ӧ˲������������������ƶ�����ɻ����� |
| C����Ӧ�������仯��˵����Ҫ�ǻ�ѧ��ת��Ϊ���ܺͶ��� |
| D���ڷ�Ӧ�и������ֻ������������ |
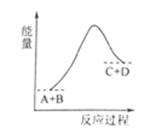
 C+D�������仯��ͼ��ʾ������˵����ȷ����
C+D�������仯��ͼ��ʾ������˵����ȷ����