��Ŀ����
��֪����1 mol C��H����Ҫ��������414.4 kJ������1 mol C��C����Ҫ��������347.4 kJ������1 mol C===C������ų�����615.3 kJ������1 mol H��H������ų�����435.3 kJ��ij�л���ֽ�ķ�Ӧ�ɱ�ʾΪ��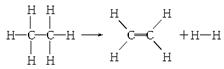
���ڷ�Ӧ��������1 mol���飬���йظ÷�Ӧ��˵����ȷ����(����)
| A���÷�Ӧ�ų�251.2 kJ������ | B���÷�Ӧ����251.2 kJ������ |
| C���÷�Ӧ�ų�125.6 kJ������ | D���÷�Ӧ����125.6 kJ������ |
D
����������������ݷ�Ӧ������ܵĹ�ϵ���÷�Ӧ����ЧӦ=��Ӧ��ϼ�ʱ���յ�����-������ɼ�ʱ�ų�������="414.4" kJ��6+347.4 kJ-��4��414.4 kJ+615.3 kJ+435.3 kJ��=125.6kJ>0��˵���÷�Ӧ���ȣ����Դ�ѡD��
���㣺���黯ѧ��Ӧ����ЧӦ�ļ���

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д����þ�γɵĻ�����ˢ���˽��������ﳬ���¶ȵ����¼������ͼ�Ǹû�����ľ���ṹ��Ԫ��þԭ�Ӽ��γ������������������������滹����һ��þԭ�ӣ�6����ԭ��λ�������IJ����ϣ���û�����Ļ�ѧʽ�ɱ�ʾΪ( )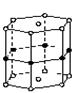
| A��MgB | B��Mg3B2 |
| C��MgB2 | D��Mg2B3 |
��֪���з�Ӧ���Ȼ�ѧ����ʽ��6C(s)+5H2(g)+3N2(g)+9O2(g)=2C3H5(ONO2)3(l) ��H1
2 H2(g)+O2(g)=2H2O(g) ��H2
C(s)+O2(g)=CO2(g) ��H3
��Ӧ4C3H5(ONO2)3(l)=12CO2(g)+10H2O(g)+O2(g)+6N2(g)�ġ�HΪ�� ��
| A��12��H3+5��H2��2��H1 | B��2��H1��5��H2��12��H3 |
| C��12��H3��5��H2��2��H1 | D����H1��5��H2��12��H3 |
��֪��ӦA+B=C+D�������仯��ͼ��ʾ������˵����ȷ���� �� ��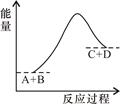
| A���÷�ӦΪ���ȷ�Ӧ |
| B���÷�ӦΪ���ȷ�Ӧ |
| C����Ӧ�������������������������� |
| D���÷�Ӧֻ���ڼ��������²��ܽ��� |
��֪��2CO(g)+O2(g)=2CO2(g) ��H= ��566 kJ?mol��1
Na2O2(s)+CO2(g) = Na2CO3(s)+1/2O2(g) ��H= ��226 kJ?mol��1
���б�����ȷ����(NA��������٤����������ֵ) ( )
| A��1molCOȼ��ʱ�ų�������Ϊ283 kJ?mol��1 |
| B��Na2O2(s)+CO(g) = Na2CO3(s)��H= ��509 kJ?mol-1 |
| C��Na2O2(s)��CO2(g)��Ӧ�ų�226 kJ����ʱ����ת����Ϊ2NA |
| D��Na2O2��Na2CO3���������Ӹ����Ȳ�ͬ |
��֪����1 mol C��H����Ҫ��������414.4 kJ������1 mol C��C����Ҫ��������347.4 kJ������1 mol C===C������ų�����615.3 kJ������1 mol H��H������ų�����435.3 kJ��ij�л���ֽ�ķ�Ӧ�ɱ�ʾΪ��
���ڷ�Ӧ��������1 mol����(��Ӧ��)�����йظ÷�Ӧ��˵����ȷ����(����)
| A���÷�Ӧ�ų�251.2 kJ������ | B���÷�Ӧ����251.2 kJ������ |
| C���÷�Ӧ�ų�125.6 kJ������ | D���÷�Ӧ����125.6 kJ������ |
��֪25 �桢101 kPa�£����з�Ӧ
C(ʯī) + O2(g) === CO2(g) ��ȼ��1 mol C(ʯī)����393.51 kJ��
C(���ʯ) + O2(g) === CO2(g)��ȼ��1 mol C(���ʯ)����395.41 kJ��
���Եó��Ľ�����
| A�����ʯ��ʯī�ȶ� | B��1 molʯī�����е�������1 mol���ʯ�� |
| C�����ʯת���ʯī�������仯 | D��ʯī�ͽ��ʯ����̼��ͬλ�� |
��101kPa 25��ʱ��1.0g����������ȫȼ������Һ̬ˮʱ�ų�����52.0kJ��������ȼ�յ��Ȼ�ѧ����ʽΪ
A��C2H6(g) �� 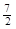 O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1 O2(g)��2CO2(g) ��3H2O(l)��H =��1560kJ��mol��1 |
| B��2C2H6(g) �� 7O2(g)��4CO2(g) ��6H2O(g)��H =��1560kJ��mol��1 |
| C��2C2H6(g) �� 7O2(g)��4CO2(g) ��6H2O(l)��H =��3120 kJ��mol��1 |
D��C2H6(g) ��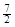 O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1 O2(g)��2CO2(g) ��3H2O(l)��H =��52.0kJ��mol��1 |
�������һ�����ᣬ��������ʴ��������֪25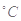 ʱ��
ʱ��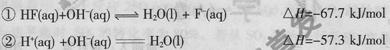
��20Ml0.lmol/L��������еμ�0.lmol/L��NaOH V mL������˵����ȷ����
A�������ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��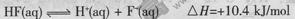 |
B����V="20" mLʱ����Һ�У� |
C����V="20" mLʱ����Һ�У� |
D����v>0ʱ����Һ��һ������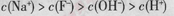 |