��Ŀ����
18������˵����ȷ���ǣ�������
| A�� | ��ϵͳ����������ʱ����ͼ1���������������2��4��6-����-3-�һ����� | |
| B�� | �л��ͼ2����һ�ַ�����ͬ���칹���ܷ���������Ӧ | |
| C�� | �߷��ӻ����ͼ3�����䵥��Ϊ�Ա���������Ҵ� | |
| D�� | ǰ�������ǹ��ұ����İ��������鼫������֮�ƣ���֪�ò����Ի�ѧҩƷ���У��Ҵ�������������Һ�����Խ������������ﵽ������Ŀ�� |
���� A���������ѡ���̼����
B�������Ͷ�Ϊ4��ͬ���칹���в�����ȩ����
C������Ϊ�Ա���������Ҷ�����
D���Ҵ�ʹ�����ʱ��ԣ�
��� �⣺A�������������Ӧ��2��4��6-����-3-�һ����飬��A��ȷ��
B�������Ͷ�Ϊ4���纬�б�������ͬ���칹���в�����ȩ������B����
C������Ϊ�Ա���������Ҷ�������C����
D���Ҵ�����Һ��������Ϊ����ʹ�����ʱ��ԣ��������ǽ�����������������������Һ���Խ������������ﵽ������Ŀ�ģ���D����
��ѡA��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬ע������л���Ĺ����ŵ����ʣ�Ϊ��������Ŀ�Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
6��NA���������ӵ�����������˵����ȷ���ǣ�������
| A�� | 5.6 g��������������ȫ��Ӧת�Ƶĵ�����Ϊ0.2NA | |
| B�� | 100 mL 2.0 mol•L-1�������������Һ�������Ӿ�Ϊ0.2NA | |
| C�� | ��״���£�22.4 L CO2��22.4 L H2O����ԭ������Ϊ3 NA | |
| D�� | ����ֱ�Ϊ1.0L pH=2��������������Һ������������Ϊ0.01NA |
7��500mL NaNO3 ��Cu��NO3��2�����Һ��c��NO3- ��=6 mol/L����ʯī���缫������Һ����ͨ��һ��ʱ����������ռ�������22.4L����״���£����ٶ�������Һ�����Ϊ500mL������˵����ȷ���ǣ�������
| A�� | ԭ�����Һ��c��Na+ ��=6mol/L | B�� | ������Һ��c��H+��=4mol/L | ||
| C�� | �����������й�ת��8mol���� | D�� | ����õ���Cu�����ʵ���Ϊ2mol |
13�������Ԫ�����ڱ���һ���֣���ش��й����⣺
��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ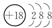 ��
��
��2���������γ��������������Ԫ����������Ԫ�����ƣ���д����Ԫ�صĵ����������������ˮ���ﷴӦ�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2����
��3���١��ܡ��ݡ��ޡ��ߡ�������Ԫ�ص�����������ˮ�����У������Լ���������ǿ��˳������Ϊ���û�ѧʽ��ʾ��KOH��Mg��OH��2��Al��OH��3��H2CO3��H2SO4��HClO4��
��4����Ԫ�����Ԫ�����ߺ˵����֮����26��
��5����д���ڵ��⻯����������Ļ�ѧ����ʽ4NH3+5O2 4NO+6H2O��
4NO+6H2O��
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
 ��
����2���������γ��������������Ԫ����������Ԫ�����ƣ���д����Ԫ�صĵ����������������ˮ���ﷴӦ�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2����
��3���١��ܡ��ݡ��ޡ��ߡ�������Ԫ�ص�����������ˮ�����У������Լ���������ǿ��˳������Ϊ���û�ѧʽ��ʾ��KOH��Mg��OH��2��Al��OH��3��H2CO3��H2SO4��HClO4��
��4����Ԫ�����Ԫ�����ߺ˵����֮����26��
��5����д���ڵ��⻯����������Ļ�ѧ����ʽ4NH3+5O2
 4NO+6H2O��
4NO+6H2O��
7�����������У�һ�ȴ����ͬ���칹�����������ǣ�������
| A�� | �춡�� | B�� | 2-������ | C�� | ���� | D�� | 3-�һ����� |
 ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�CH2CH=CH2+CO+H2$��_{��}^{����}$CH3CH3CH2CHO$��_{��}^{H_{2}����}$CH3CH2CH2CH2OH��
ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�CH2CH=CH2+CO+H2$��_{��}^{����}$CH3CH3CH2CHO$��_{��}^{H_{2}����}$CH3CH2CH2CH2OH��
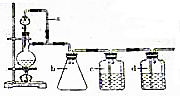
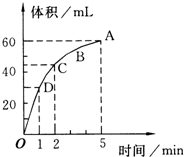 ����0.1mol��MnO2��ĩ��50mL�����������Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��5���ӷ�Ӧ��ɣ����ش��������⣺
����0.1mol��MnO2��ĩ��50mL�����������Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��5���ӷ�Ӧ��ɣ����ش��������⣺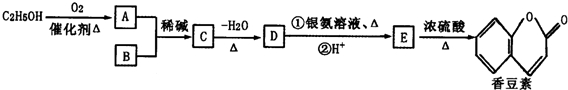
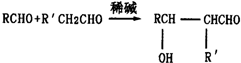 ��R��R��Ϊ��������ԭ�ӣ�
��R��R��Ϊ��������ԭ�ӣ�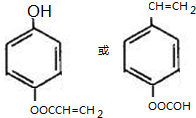 ��
��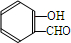 $\stackrel{NaOH}{��}$
$\stackrel{NaOH}{��}$ 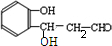 ��
�� CO���廹ԭFeO������Եõ�Fe���壬���Ļ����������Ҫ��Ӧ�ã�
CO���廹ԭFeO������Եõ�Fe���壬���Ļ����������Ҫ��Ӧ�ã�