��Ŀ����
14���������й㷺����;��
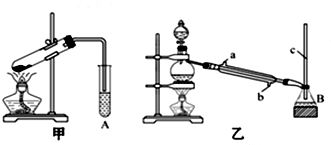
��1��ʵ���ҳ�����ͼ1��ʾװ����ȡ���ռ�������
��ʵ������ȡ������Ӧ�Ļ�ѧ����ʽ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��ͼ�з������ռ�������װ�ÿ�ѡ��C������ĸ��ţ���
��β������װ����ʹ�õ���©���������Ƿ�ֹ��Һ������
��2����ͼ2װ�ý�����Ȫʵ��
���ռ�����ʱӦ��a���a����b������������
��ʵ����B����ƿ�ڳ�����Ȫ����ˮ��Һ������ȫ����������ƿ��˵��ˮ��Һ���ܳ�����ƿ��ԭ����ƿ���ռ��İ������������в��ֿ�����
���� ��1����ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ�Ʊ��������ڰ������ܶȱȿ������ܶ�С������Ҫ�������ſ������ռ�������
�۰�����������ˮ������©���ռ���ܷ�ֹ������
��2���ٰ������ܶ�С�ڿ�����Ӧ�������ſ������ռ���
�ڰ����������ſ������ռ����ռ��İ����л��в��ֿ�����
��� �⣺��1������κͼ��ܷ�Ӧ���ɰ�����ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ�Ʊ���������Ӧ�Ļ�ѧ����ʽΪ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ڰ�����������ˮ���ܶȱȿ���С�������ռ�������ѡ�������ſ����������ݽ�������Ӧ����Cװ�ã�
�ʴ�Ϊ��C��
�۰�����������ˮ������©���л������ã������ܳ�ֱ������ҷ�ֹ������
�ʴ�Ϊ����ֹ��Һ������
��2���ٰ������ܶ�С�ڿ�����Ӧ�������ſ������ռ����ռ�����ʱӦ��a��������
�ʴ�Ϊ��a��
�ڿ����еĵ���ϡ������������ˮ���������ſ������ռ��������ռ��İ����л��в��ֿ�����������Ȫˮ��Һ������ȫ����������ƿ��
�ʴ�Ϊ����ƿ���ռ��İ������������в��ֿ�����
���� ���⿼���˰��������ʺ���ȡ��������κͰ����������ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | �γ����Ӽ����������Ӽ�ֻ���ھ��������� | |
| B�� | HF��HCl��HBr��HI�����ȶ��Ժͻ�ԭ�Ծ����μ��� | |
| C�� | �������ڷǽ���Ԫ�غ���������Դ�����������ǿ | |
| D�� | NH5�е�����ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ��Ӳ�ṹ��1molNH5�к���4NA��N-H����NA��ʾ�����ӵ�������ֵ�� |
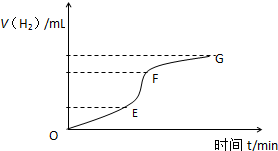 �ô�����п����ϡ���ᷴӦ��ȡ��������ش�
�ô�����п����ϡ���ᷴӦ��ȡ��������ش���1����ͼΪ�����뷴Ӧʱ���ϵͼ�������ж�EF�λ�ѧ��Ӧ������죬����ԭ���Ǹ÷�Ӧ�Ƿ��ȷ�Ӧ��������Һ�Ľ��У���Һ�¶����ߣ���ҺŨ����Ȼ��С�����¶�Ӱ�����Ũ��Ӱ�죬���Է�Ӧ���ʿ죮
��2��ijѧ����100mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼���£��ۼ�ֵ�����������ת��Ϊ��״�����������
| ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
| ���������mL�� | 50 | 120 | 232 | 290 | 310 |
��3��Ϊ�˼���������Ӧ�����ʣ�������Һ�м����������ʣ�����Ϊ���е���AC��
A������ˮ B���Ȼ��ƹ��� C���Ȼ�����Һ D��Ũ����
��4���������������⣬����Ϊ�����Բ�ȡ��Щ��ʩ��������ѧ��Ӧ���ʣ������ٻش�һ�֣������¶ȣ�
| A�� | �÷�Ӧ������Cl2��ǿ������ | B�� | ���ܵ�©�������ͻ�������� | ||
| C�� | �÷�Ӧ���ڸ��ֽⷴӦ | D�� | ����1molN2��6mol����ת�� |
| A�� | �������ڣ���֬����Ҫ��Ӧ����֬��ø����ˮ��Ϊ��֬������� | |
| B�� | �����˿������˿����ά�أ��ķ���֮һ�����գ����ս���ë��ζ���Dz�˿ | |
| C�� | ũ���˽������ؽ��ոѷ��Ͳ����������ɼ�������մ����Ļ�����Ⱦ | |
| D�� | ��������������������ҪԪ�أ��ʺ������ķ�ˮ��ֱ���ŷŵ������� |
| A�� | c��Na+��+c��H+��=c��OH-��+c��HC2O4-��+c��C2O42-�� | |
| B�� | c��Na+��=2c��HC2O4-��+2c��H2C2O4��+2c��C2O42-�� | |
| C�� | c��OH-��-c��HC2O4-��=c��H+��+2c��H2C2O4�� | |
| D�� | c��Na+����c��C2O42-����c��OH-����c��HC2O4-����c��H+�� |
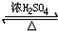 HCO18OCH3+H2O��
HCO18OCH3+H2O�� ��
��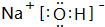 ��
�� �ȣ�һ�ֹ��ۻ�����ĵ���ʽΪ
�ȣ�һ�ֹ��ۻ�����ĵ���ʽΪ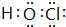 �ȣ�
�ȣ�