��Ŀ����
A��B��C��D���ֿ������Σ�֪�������ӷֱ���Na+��Ba2+��Cu2+��Ag+ �е�ijһ�֣�
�����ӷֱ���Cl-��SO42-��CO32-��NO3- �е�ijһ�֡���������ʵ�飺
�� �������θ�ȡ�������ֱ�����ʢ��5 mL����ˮ����֧�Թ��У�ֻ��B����Һ����ɫ��
�� �ֱ���4֧�Թ��м���2 mLϡ���ᣬ����A����Һ�в�����ɫ������C����Һ���н϶����ݲ�������D����Һ����������
��1������������ʵ���ƶ��������εĻ�ѧʽ�ֱ�Ϊ��
A ��B ��C �� D ��
(2)д��ʵ�鲽������漰�������з�Ӧ�����ӷ���ʽ�� ��
��1��A��AgNO3��B��CuSO4��C��Na2CO3�� D��BaCl2����1�֣�
��2��Ag++Cl-��AgCl����CO32-+2H+��CO2��+H2O����2�֣�
���������������1��B�ε���Һ����ɫ��˵��B���к���Cu2+���ֱ���4֧�Թ��м���2mLϡ���ᣬ����A����Һ�в�����ɫ������˵��A���к���Ag+��C����Һ���н϶����ݲ�����˵��C���к���CO32-������ΪA��B��C��D�����ξ�Ϊ�������Σ���A��ΪAgNO3��C��ΪNa2CO3��B��ΪCuSO4��D��ΪBaCl2��
��2��ʵ�鲽������漰�������з�Ӧ�����ӷ���ʽ��Ag++Cl-��AgCl����CO32-+2H+��CO2��+H2O��
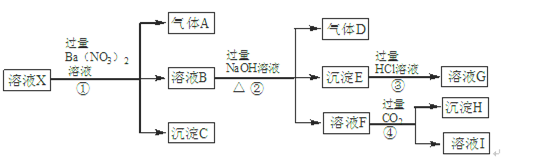
���㣺�������ӹ��桢���Ӽ����Լ����ӷ���ʽ����д��

ij�����Һ�п��ܺ��е��������±���ʾ��
| ���ܴ������е������� | H+��Ag+��Mg2+��Al3+��NH ��Fe3+ ��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO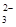 ��AlO ��AlO |
Ϊ̽����ɷ֣�����������̽��ʵ�顣
��1��̽��һ��
��ͬѧȡһ�����Ļ����Һ����������μ�������������Һ���������������ʵ���(n)��������� ������Һ�������V���Ĺ�ϵ��ͼ��ʾ��
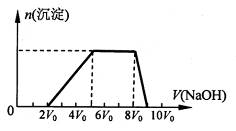
�ٸ���Һ��һ�����е���������______________�����Ӧ���ʵ���Ũ��֮��Ϊ________��һ�������ڵ���������_____________��
����д���������ٹ����з�����Ӧ�����ӷ���ʽ_____________________________��
��2��̽������
��ͬѧ������Һ�к��д�����Cl-��Br-��I-������1 L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ�� ������ش��������⣺
| Cl2���������״���� | 5.6 L | 11.2 L | 22.4 L |
| n (Cl-) | 2.5 mol | 3.0 mol | 4.0 mol |
| n (Br-) | 3.0 mol | 2.8 mol | 1.8 mol |
| n (I-) | x mol | 0 | 0 |
�ٵ�ͨ��Cl2�����Ϊ5.6 Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ_______________��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ______________________��