��Ŀ����
�������ȣ�ClO2���ڳ�������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11��0�棬������ˮ����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��ʱ��Ӧ�Ƶá�ijѧ��������ͼ��ʾװ��ģ�ҵ��ȡ���ռ�ClO2�����ڸ÷�Ӧ�����ȷ�Ӧ������������������ڸ÷�Ӧ�Է�����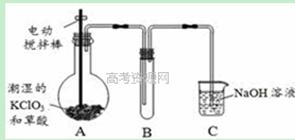
��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��Bװ�ñ�����ڱ�ˮԡ�У���ԭ���� ��
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2���벹���NaClO2��Һ���Ƶ�NaClO2����IJ������裺�� ���� ����ϴ�ӣ��ܸ��
��4��ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00 mL��ϡ�ͳ�100.00 mL��������ȡV1 mL�������뵽��ƿ�У�
����2����ϡ�������������pH��2.0������������KI���壬����Ƭ�̣�
����3���������ָʾ������c mol/L Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪2 Na2S2O3 + I2��Na2S4O6 + 2NaI��
������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ����������У� ��
��д������2�з�����Ӧ�����ӷ���ʽ ��
��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����
�����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ����ⶨ��� ��
���ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ��������ⶨ��� ��
���ƫ�ߡ���ƫ�͡����䡱 ��
��1��2KClO3+H2C2O4 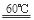 K2CO3+CO2��+2ClO2��+H2O;
K2CO3+CO2��+2ClO2��+H2O;
��2���¶ȼ�; ʹClO2������������ٻӷ�;
��3���������ᾧ����д������Ũ�����ᾧ�����֣����ڳ��ȹ��ˣ�
��4�� �� 100ml ����ƿ����ͷ�ιܣ���2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O��
��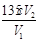 ����ƫ�ߣ�ƫ�͡�
����ƫ�ߣ�ƫ�͡�
���������������1��������֪��Ϣ�ɵ÷���ʽΪ��2KClO3+H2C2O4 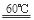 K2CO3+CO2��+2ClO2��+H2O;��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ����¶ȼƣ�Bװ�ñ�����ڱ�ˮԡ�У���Ϊ��ʹClO2������������ٻӷ�����3������NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2�����Դ�NaClO2��Һ���Ƶ�NaClO2����IJ��������Ǣ������ᾧ��Ȼ��ڳ��ȹ��ˣ���ϴ�ӣ��ܸ���Ӷ��õ�NaClO2���塣��4��������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ�����������100ml ����ƿ����ͷ�ιܣ���ClO2�������ԣ���I-�л�ԭ�ԣ����������������·���������ԭ��Ӧ�����ݵ����غ㡢ԭ���غ��֪������2�з�����Ӧ�����ӷ���ʽΪ2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O�����ɷ���ʽ�ù�ϵʽΪ2ClO2��5I2��10Na2S2O3��n(Na2S2O3)=" c" mol/L��V2��10-3L������ϡ�ͺ��V1 mL��Һ�к��е�ClO2�����ʵ���Ϊ2 cV2��10-4mol.��ԭ��Һ10ml��ϡ�ͺ��100m����Һ�к��е�ClO2�����ʵ���Ϊ(2 cV2�� V1)��10-2mol.��ԭ��Һ��Ũ��Ϊ��c(ClO2)= (2 cV2��V1)��10-2mol��0.01L=(2 cV2��V1)mol/L=
K2CO3+CO2��+2ClO2��+H2O;��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ����¶ȼƣ�Bװ�ñ�����ڱ�ˮԡ�У���Ϊ��ʹClO2������������ٻӷ�����3������NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2�����Դ�NaClO2��Һ���Ƶ�NaClO2����IJ��������Ǣ������ᾧ��Ȼ��ڳ��ȹ��ˣ���ϴ�ӣ��ܸ���Ӷ��õ�NaClO2���塣��4��������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ�����������100ml ����ƿ����ͷ�ιܣ���ClO2�������ԣ���I-�л�ԭ�ԣ����������������·���������ԭ��Ӧ�����ݵ����غ㡢ԭ���غ��֪������2�з�����Ӧ�����ӷ���ʽΪ2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O�����ɷ���ʽ�ù�ϵʽΪ2ClO2��5I2��10Na2S2O3��n(Na2S2O3)=" c" mol/L��V2��10-3L������ϡ�ͺ��V1 mL��Һ�к��е�ClO2�����ʵ���Ϊ2 cV2��10-4mol.��ԭ��Һ10ml��ϡ�ͺ��100m����Һ�к��е�ClO2�����ʵ���Ϊ(2 cV2�� V1)��10-2mol.��ԭ��Һ��Ũ��Ϊ��c(ClO2)= (2 cV2��V1)��10-2mol��0.01L=(2 cV2��V1)mol/L=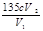 g/L. �����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ�������ĵ�Na2S2O3���ƫ��ʹ�òⶨ���ƫ�ߣ����ζ���ʼ���Ӷ�������ʼ����ƫ�ζ��յ�ʱ��ȷ�����������ĵ�Na2S2O3���ƫС���ⶨ���ƫ�͡�
g/L. �����ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ�������ĵ�Na2S2O3���ƫ��ʹ�òⶨ���ƫ�ߣ����ζ���ʼ���Ӷ�������ʼ����ƫ�ζ��յ�ʱ��ȷ�����������ĵ�Na2S2O3���ƫС���ⶨ���ƫ�͡�
���㣺���黯ѧ��Ӧԭ����������ʹ�á����ʵ���ȡ������������ѧ����ʽ�����ӷ���ʽ����д��Ũ�ȵļ����֪ʶ��

��1������
| ���ʣ����ʣ� | ��ȥ���� �����Լ��� | ���ӷ���ʽ ���������ӷ���ʽ����д��ѧ����ʽ�� |
| Fe(Al) | | |
| SiO2��CaCO3�� | | |
| CO2(HCl) | | |
| NaHCO3��Һ��Na2CO3�� | | |
| Na2CO3��NaHCO3�� | | |
| Al2O3��Al(OH)3�� | | |
��2������������ͨ�뵽�Ȼ�����Һ��δ��������������ͨ�������Һ�еμ���ˮ��ˮ����������ɫ�����������ó����ijɷֱַ�Ϊ �� ����ֱ�д��������Ӧ�����ӷ���ʽ �� ��
��ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ��Իش���������
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
��. �����������壨FeC2O4��2H2O����̼��﮺Ͷ�������������и��·�Ӧ���Ʊ�﮵�ص��������Ϲ�������ﮣ�Li2FeSiO4��������������������������н������ط������������ͼ��ʾ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�����,��ش��������⣺
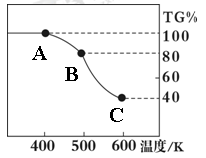
��5����������������̼Ԫ�صĻ��ϼ�Ϊ��
��6��A��B������Ӧ�Ļ�ѧ����ʽΪ ��
��7����ȷ�о�������B��Cʵ���Ƿ��������еģ�ÿһ��ֻ�ͷ�һ�����壬�ڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����һ���ͷŵ����廯ѧʽΪ�� ���ͷŵڶ�������ʱ����Ӧ�Ļ�ѧ����ʽΪ ��
ijС�������֪:������I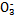 >Fe3+>I2,3Br2+6FeCl2
>Fe3+>I2,3Br2+6FeCl2 2FeBr3+4FeCl3;I2+2S2
2FeBr3+4FeCl3;I2+2S2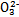
 S4
S4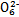 +2I-;CuI��һ�ְ�ɫ����(Ksp=1.3��1
+2I-;CuI��һ�ְ�ɫ����(Ksp=1.3��1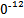 )��
)��
��.��С��Ϊȷ��һ�ݼӵ���(���ܺ���KIO3��KI��Mg2+��Fe3+)�ijɷ�,��ƶ���ʵ����Բ������֤��
(1)ʵ���������:
| ʵ�鲽�� | ʵ����̺����� | ��Ӧ���� | |
| ����1 | ȡһ��������,����������ˮ�ܽ�,����ϡ�����ữ,��������Һ��Ϊ3�� | _____________ | |
| �� �� 2 | �ڢٷ� ��Һ | 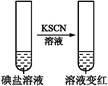 | �����п϶����������� |
| �ڢڷ� ��Һ | 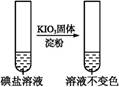 | _____________ | |
| �ڢ۷� ��Һ | 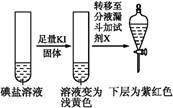 | XΪ������(�ѧʽ) | |
(2)�õ����п϶���������������������(�û�ѧʽ�����ӷ��ű���)��
(3)������Ϣ�ƶ�Fe3+��S4
 ��I2��Br2����������ǿ������˳������������������������
��I2��Br2����������ǿ������˳������������������������ (4)�ڢ۷���Һ�м�������KI�����,��Ӧ�����ӷ���ʽΪ��������������������������������������
��.�á���ӵ��������ⶨ����CuCl2��2H2O���������(��������I-������Ӧ������������)�Ĵ���,��������:
ȡ0.40 g��������ˮ,�������KI����,��ַ�Ӧ,���ɰ�ɫ����������������ζ�ָʾ��,��0.100 0 mol��L-1 Na2S2O3����Һ�ζ�,����ζ��յ�ʱ,����Na2S2O3����Һ20.00 mL��
(1)�ζ��յ������������
(2)CuCl2��Һ��KI��Ӧ�Ļ�ѧ����ʽΪ��������������������������������
(3)��������CuCl2��2H2O��������������������
MnO2��һЩ���ʻ���;��ͼ������˵����ȷ���� ( )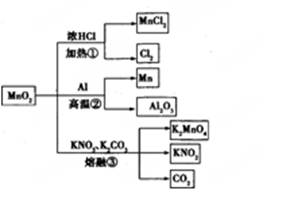
| A���١��ڡ���������Ӧ��MnO2���������� |
| B������MnO2��2 L 10 mol/LHCl���ȣ�������5 mol C12 |
| C����Ӧ��������1 mol Al2O3����Ӧ������ת��12 mol���� |
| D����Ӧ����K2CO3��KNO3�Ļ�ѧ��������Ϊ1 |
���ݱ�����Ϣ�жϣ�����ѡ����ȷ���� �� ��
| ��� | ��Ӧ�� | ���� |
| �� | KMnO4��H2O2��H2SO4 | K2SO4��MnSO4���� |
| �� | Cl2��FeBr2 | FeCl3��FeBr3 |
| �� | MnO4������ | Cl2��Mn2+���� |
B���ڢ��鷴Ӧ��Cl2��FeBr2�����ʵ���֮�ȴ��ڻ����1:2
C���ڢ��鷴Ӧ������1mol Cl2��ת�Ƶ���5mol
D����������ǿ����˳��ΪMnO4��>Cl2> Br2> Fe3+
ij�Ͻ�(����ͭ����)��ͭ���������ʵ���֮��Ϊymol������Cu�����ʵ�������Ϊa ������ȫ��Ͷ��50mLbmol��L��1��������Һ�У�����ʹ���ַ�Ӧ(����NO��Ψһ�Ļ�ԭ����)������˵������ȷ����
| A����������ʣ�࣬����Һ���ٵ�����������ֿ�ʼ�ܽ� |
| B��������ȫ���ܽ⣬����Һ�в�һ������Fe3�� |
| C��������ȫ���ܽ⣬�Ҳ���336mL����(��״��)����b=0.3 |
| D������Һ�н�������ֻ��Fe3����Cu2��ʱ����a��b�Ĺ�ϵΪ��b��80y(1��a/3) |
��ʯ�н�ĺ����ܵ�,����������൱�ȶ�,ֻ����ʪ��ұ�𡪡��軯������,������ϡ��NaCN��Һ�����ѷ���Ŀ�ʯ,�������Ľ������Һ��,Ȼ���ý���п����ԭ���������Һ���û��������䷴Ӧԭ��Ϊ
��4Au+8NaCN+O2+2H2O 4Na[Au(CN)2]+4NaOH;
4Na[Au(CN)2]+4NaOH;
��2Na[Au(CN)2]+Zn 2Au+Na2[Zn(CN)4]��
2Au+Na2[Zn(CN)4]��
�����й��軯���������˵������ȷ����( )
| A��Na[Au(CN)2]������ˮ |
| B��������Na[Au(CN)2]�н�Ԫ�صĻ��ϼ�Ϊ+1 |
| C����Ԫ���ڵؿ������Ի���̬����ʽ���� |
| D�������������к���Ԫ�ص�����ʼ���������� |