��Ŀ����
����Ŀ��NA���������ӵ�������ֵ������˵����ȷ����
A.2NA��HCl������44.8 L H2��Cl2�Ļ������������ԭ����Ŀ��Ϊ4NA��
B.32gCu������Ũ��ϡ����ֱ�ԭΪNO2��NO��Ũ��ϡ����õ��ĵ�������ΪNA��
C.���ʵ���Ũ�Ⱦ�Ϊ1mol/L��NaCl��MgCl2�����Һ�У�����Cl�D����ĿΪ3NA��
D.1mol D318O+������D����![]() ���к��е�������Ϊ10 NA��
���к��е�������Ϊ10 NA��
���𰸡�B
��������
A������������������״̬����ȷ���������ʵ��������㣬���HCI��ԭ����Ŀ�Ƿ���ͬ�����㣬A����
B��32gCu�����ʵ���0.5mol��ʧȥ�ĵ�������ΪNA��Ũ���ᱻ��ԭΪ����������ϡ���ᱻ��ԭΪNO�����ݵ�ʧ�������غ㣬Ũ��ϡ����õ��ĵ�������ΪNA��B��ȷ��
C����Һ�������ȷ������Һ�к��е������Ӹ��������㣬C����
D��D318O+�к�13�����ӣ���1molD318O+�к�13NA�����ӣ�D����
��ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������һ����Ҫ��Ԫ�أ��������ϳ�NH3�� HNO3��һϵ����Ҫ������Ʒ��
(1)�ڱ�״���£�1���ˮ�ܽ�700���������������Һ���ܶ�Ϊ0.9g/mL����ð�ˮ�����ʵ���Ũ����_______��С�������һλ����
(2)�ϳɰ���ԭ����N2��H2ͨ�����Խ�̿��ˮ�Ϳ���Ϊԭ������ȡ�ġ�����Ҫ��Ӧ�ǣ�
��2C + O2 �� 2CO ��C + H2O(g) �� CO + H2 ��CO + H2O(g) �� CO2 + H2
ij�������н���̿��H2O(g)�Ϳ������������N2��O2�������Ϊ4:1����ͬ����Ϸ�Ӧ������������ᆳ������������±���
���� | CO | N2 | CO2 | H2 | O2 |
�����m3������״���� | x | 20 | 12 | 60 | 1.0 |
�����μӷ�Ӧ��H2O(g)�������������V(H2O)/V(����)=__________�ϱ���x=________m3��ʵ��������___________ kg��̿��
(3)��ҵ��������ʱβ�������ִ����Ա������Ⱦ������NaOH��Һ���գ�����ʱ������Ӧ��
��2NO2 + 2NaOH��NaNO3 + NaNO2 + H2O
��NO + NO2 + 2NaOH��2NaNO2 + H2O
�ֽ�22.4������״����NOx��ֻ��NO��NO2�����������ɷ֣����建��ͨ������NaOH��Һ�У���ַ�Ӧ������ȫ�������ա���Ӧ�в���NaNO3��NaNO2�����ٿˣ����ú�x�Ĵ���ʽ��ʾ��____________________
����Ŀ��̼��������ҹ���Ҫ�ĵ���Ʒ��֮һ���������������������ӷ���ʧ��Ϊ�˼�����������ȷ�����ʩ����������ⶨ�京������
��.ijѧ�������һ���Բⶨ������̼������Ӳⶨ�������ķ���������Ʒ����Բ����ƿ�У�
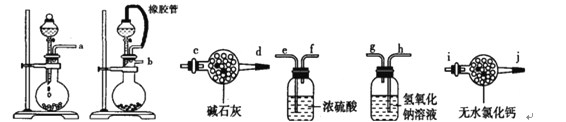
(1)��ѡ���Ҫ��װ�ã���������������˳��Ϊ________________��
(2)��Һ©���е�Һ�����ʺϵ���___________��
A��ϡ���� B��ϡ���� C��Ũ���� D����������
(3)��������װ��������____________________________________��
����������гɷ��ǣ�NH4��2SO4��������ü�ȩ���ⶨ����������ȩ���ǻ��ڼ�ȩ��һ������������ã������൱�����ᣬ��ӦΪ2��NH4��2SO4+6HCHO����CH2��6N4 +2H2SO4 + 6H2O,���ɵ��������������Ʊ���Һ�ζ����Ӷ��ⶨ���ĺ������������£�
(4)�ò�������ȡ���壨NH4��2SO4��Ʒ0��6g���ձ��У�����Լ30mL����ˮ�ܽ⣬�������100mL��Һ����________ȷȡ��20��00mL����Һ����ƿ�У�����18%���Լ�ȩ��Һ5mL������5min����1~2��________ָʾ������֪�ζ��յ��pHԼΪ8��8������Ũ��Ϊ0.08mol/L�������Ʊ���Һ�ζ����������±���
�ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
1 | 1��20 | 16��21 |
2 | 3��00 | 18��90 |
3 | 4��50 | 19��49 |
��ζ��յ�ʱ������Ϊ______________________________���ɴ˿ɼ��������Ʒ�еĵ�����������Ϊ_________________��
(5)�ڵζ�ʵ��������ֵζ��õĵζ��ܲ��������ڳ��������ݣ��ζ���ʼʱ�����ݣ����ʵ��ⶨ�ĺ�������ʵ��ֵ________������ƫ������ƫС��������Ӱ��������