��Ŀ����
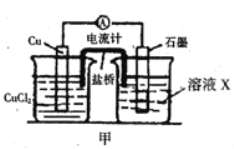
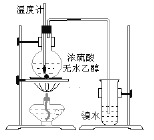
����Ŀ��ij��ѧ��ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾװ�ý����й�ʵ�顣��ش�

��1��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ:_____________��
��2��װ��D���Թܿڷ��õ����н���NaOH��Һ����������______��
��3��װ��B�������������������塣��D�������Ե�����ر�����K����ȥ�ƾ��ƣ����������ȵ����ã�A�����������������ʱB�е�������__��B��Ӧ���õ�Һ����____(�����)��
A��ˮ B������KMnO4��Һ C��Ũ��ˮ D������NaHSO3��Һ
��4��ȡһ��������ͭƬ��һ�����Ũ��Ϊ18.4 mol��L��1��Ũ�������Բ����ƿ�й��ȣ�ֱ����Ӧ��ϣ�������ƿ��ͭ�����ᶼ��ʣ�ࡣ����ʹ��ƿ��ʣ���ͭƬ�ܽ⣬������ƿ�м��������Լ��е�____������ţ���
A��HNO3 B��NaNO3 C��NaHCO3 D��Na2CO3
���𰸡�Cu+2H2SO4(Ũ) ![]() CuSO4+SO2��+2H2O ���ն�������ֹ��Ⱦ���� ���ƿ��Һ���½�������©����Һ������ D AB
CuSO4+SO2��+2H2O ���ն�������ֹ��Ⱦ���� ���ƿ��Һ���½�������©����Һ������ D AB
��������
ͭ��Ũ���ᷴӦ��������ͭ�����������ˮ�����ɵĶ����������C���ռ���Ȼ�����D��ʹƷ����ɫ�����������ж�������������������Կ���������������Һ���ա�����Ķ���������Դ�����B�С�
��1��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��Cu+2H2SO4(Ũ) ![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��2������������Ⱦ������Ҫ���д���������ֱ���ŷŵ������У������������������壬�������ƿ������ա��ʴ�Ϊ�����ն�������
��3��װ��B�������������������壬������������������������ˮ�������ڱ���NaHSO3��Һ����ѡ��NaHSO3��Һ��A���������������Bװ�ã�B����ѹ����Һ��ѹ�볤��©������ʱ�Լ�ƿ��Һ���½�������©����Һ���������ʴ�Ϊ���Լ�ƿ��Һ���½�������©����Һ��������D��
��4����Ӧһ��ʱ��������ϡ���������ܽ�ͭ������Ҫ������������Ե������ܽ�ͭ���������ǿ�����ԣ������ܽ�ͭ�������Ƽ���A�У���Һ�����ԣ��������������������ǿ�����ԣ�Ҳ���ܽ�ͭ���ʴ�Ϊ��AB��

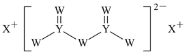
����Ŀ�������������к���![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() �ȣ�Ϊ�˳��������Դ��һ�������������������̷���
�ȣ�Ϊ�˳��������Դ��һ�������������������̷���![]() ���Ļ����������£�
���Ļ����������£�
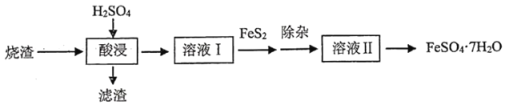
�ش��������⣺
��1�������Ҫʹ�ý�Ũ�����Ტ���ȣ�Ŀ����________________________________������![]() ��
��![]() �����ᣬ�����ʵ���Ũ��
�����ᣬ�����ʵ���Ũ��![]() _____________
_____________![]() ������3λ��Ч���֣���
������3λ��Ч���֣���
��2��������![]() �⣬�����ܺ���__________����Һ���豣��
�⣬�����ܺ���__________����Һ���豣��![]() ��ԭ����__________��
��ԭ����__________��
��3����![]() ��ԭʱ������������Ϊ
��ԭʱ������������Ϊ![]() ��������Ҫ��Ӧ�����ӷ���ʽ��___________________������÷�Ӧ�Ƿ���ȫ���������õ��Լ���___________________��
��������Ҫ��Ӧ�����ӷ���ʽ��___________________������÷�Ӧ�Ƿ���ȫ���������õ��Լ���___________________��
��4������ʱ����ȥ��Һ�е�![]() �����˵�������_______________��
�����˵�������_______________��
A NaOH B H2S C FeS D ��ˮ
��5����֪![]() ���ڲ�ͬ�¶��µ��ܽ�Ⱥ����������������±���
���ڲ�ͬ�¶��µ��ܽ�Ⱥ����������������±���
�¶�/�� | 0 | 10 | 30 | 50 | 56.7 | 60 | 64 | 80 | 90 | |
�ܽ��/g | 14.0 | 17.0 | 25.0 | 33.0 | 35.2 | 35.3 | 35.6 | 33.0 | 30.5 | 27.0 |
�������� |
|
|
| |||||||
��Ҫ���![]() ���������Һ����еIJ����ǣ�����Ũ����___________________�����ˣ�ϴ�ӣ����
���������Һ����еIJ����ǣ�����Ũ����___________________�����ˣ�ϴ�ӣ����