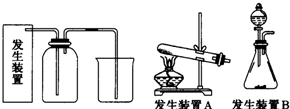
��Ŀ����
7��ʵ�������ñ�������������Ʊ�������������ʵ��װ����ͼ1��ʾ�������õ����й��������±���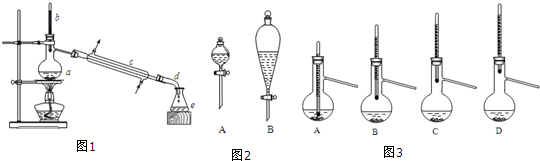
| ������ | ������ | ���������� | ������ | |
| �ܶ�/��g/cm3�� | 0.810 | 1.049 | 0.882 | 0.7689 |
| �е�/�� | 118.0 | 118.1 | 126.1 | 142 |
�����ᴿ���ٽ�����a�з�Ӧ��Ļ��Һ������e�е������ϲ���ת���Һ©���У���������ˮ��������10%̼������Һ��ˮϴ�ӣ�������ټӸ����M������һ��ʱ�����ȥM���۽����յõ��ķ�Ӧ�ֲ���ת��ϴ��������a�У����뼸����ʯ���������õ�����������7.31g��
��ش��������⣺
��1������a��������������ƿ��
��2���ڷ�ҺʱӦѡ��ͼ2װ���е�B������ţ���ʹ�ø�����ǰӦ�ȼ�©��
��3��д���Ʊ������������Ļ�ѧ����ʽ��CH3COOH+CH3CH2CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O��
��4����һ��ˮϴ��Ŀ���dz�ȥ���ἰ�������ᣮ
��5�������M������B������ţ�
A������������ B����ˮ������ C����ʯ�� D���������ƹ���
��6������۵õ��Ĵֲ�Ʒ�����ᴿʱ��ͼ3��ʾװ���У��¶ȼ�λ�ÿ��ܵ����ռ����IJ�Ʒ�л��и߷е����ʵ�װ��ΪD������ţ�
��7����ʵ�����õ��������������IJ�����C������ţ�
A��30% B��40% C��50% D��55%
���� ��1������aΪ������ƿ��
��2��AΪ���η�Һ©�������ý��м�Һʹ�ã�BΪ���η�Һ©�������ڷ�Һ����ʹ�ã���ʹ��֮ǰ��Ҫ����Ƿ�©ˮ��
��3����������������Ӧ�õ�������������ˮ��
��4����Ӧ��Ũ��������������ˮ������ӦΪ���淴Ӧ�����в���ȫ�ԣ���Ϻ���ˮϴ�ӣ�ϴȥ����������ȣ�
��5�������������ԡ����������¶����Է���ˮ�ⷴӦ����Ӧ�����Ը������
��6�������������������ᴿ���¶ȼ�ˮ����Ӧ��������ƿ֧�ܿ�λ�ã��ⶨ��Ҫ��ֵ��¶ȣ��¶�Խ�ߵ������������ƿ�е�λ�����Խ�ߣ�
��7���������ᡢ�����������������й������㣬���ݲ�����������ȷ�����������������۲�����������������ʣ�
��� �⣺��1������aΪ������ƿ���ʴ�Ϊ��������ƿ��
��2��AΪ���η�Һ©�������ý��м�Һʹ�ã�BΪ���η�Һ©�������ڷ�Һ����ʹ�ã�Ӧѡ��Bװ�ý��з�Һ�����������Ͽ���ƿ�����²��л�������ʹ��֮ǰ��Ҫ����Ƿ�©ˮ��
�ʴ�Ϊ��B����©��
��3����Ũ���������������������£���������������Ӧ�õ�������������ˮ����Ӧ����ʽΪ��CH3COOH+CH3CH2CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O��
��4����Ӧ��Ũ��������������ˮ������ӦΪ���淴Ӧ�����в���ȫ�ԣ���Ϻ���ˮϴ�ӣ�ϴȥ�����벿�����ᣬ
�ʴ�Ϊ����ȥ���ἰ�������
��5��������������ˮ�γ����ᣬ��ʯ�ҡ��������ƶ��Լ��ԣ������������ԡ����������¶����Է���ˮ�ⷴӦ����Ӧ�����Ը������ˮ�����ƣ���ѡ��B��
��6�������������������ᴿ���¶ȼ�ˮ����Ӧ��������ƿ֧�ܿ�λ�ã��ⶨ��Ҫ��ֵ��¶ȣ��¶�Խ�ߵ������������ƿ�е�λ�����Խ�ߣ���ѡ��D��
��7�������������=1.049g/mL��7.2mL=7.5528g��
������9.32g��������ȫ��Ӧ�������������xg����
CH3COOH+CH3CH2CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O
60g 74g
xg 9.32g
���ԣ�60g��74g=xg��9.32g�����x=7.5568��7.5528g��������������������۵õ�����������������Ϊ��$\frac{116g��9.32g}{74g}$�������������������IJ���=��7.31g��$\frac{116g��9.32g}{74g}$����100%��50%��
��ѡ��C��
���� ���⿼���л���ĺϳ�ʵ�飬�漰��ѧ������ʹ�á���Ӧԭ����ϴ�������������ⶨ�ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

| A�� | ��Fe��NO3��2��Һ�м�ϡ���3Fe2++4H++NO3-�T3Fe3++NO��+2H2O | |
| B�� | ���Ȼ�淋�ϡ��Һ�м�������NaOH�����ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| C�� | ��NH4��2SO4��Һ�м�������Ba��OH��2��Ba2++SO42-�TBaSO4�� | |
| D�� | ��ϡ�����м���ͭƬ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O |
| A�� | 0.2mol•L-1������Һ��0.2mol•L-1��������Һ�������ϣ�C��CH3COOH��+C��H+��-C��OH-��=0.1mol•L-1 | |
| B�� | pH��ȵĢ٣�NH4��2SO4��NH4HSO4��NH4Cl��Һ��c��NH4+����С˳��Ϊ���٣��ڣ��� | |
| C�� | 0.1mol•L-1Na2CO3��Һ��c��Na+��+c ��H+��=C ��CO32-��+c��HCO3-��+c��OH-�� | |
| D�� | 0.1mol•L-1��NaHB��Һ������PH=4��c��HB-����c��H+����c��H2B����c��B2-�� |
| A�� | ��������Һ�ʹ���Ǧ��Һ����ʹ�����ʱ��� | |
| B�� | ������ʴ�ı����ǽ���ԭ��ʧ���ӱ������Ĺ��� | |
| C�� | ��ҵ����������ˮ�ࡢ�մɣ�����Ҫ��ʯ��ʯΪԭ�� | |
| D�� | ������ά���ϳ���ά���ά�����л��߷��ӻ����� |
 ���������������ܼ�����ˮ�������ϡ����ᡢ����ȵķ�����ȣ�ʵ���Һϳ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
���������������ܼ�����ˮ�������ϡ����ᡢ����ȵķ�����ȣ�ʵ���Һϳ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�Na2B4O7•10H2O+2H2SO4+12CH3OH=2NaHSO4+4��CH3O��3B+17H2O
| ���������� | �״� | |
| ��Է����� | 104 | 32 |
| �ܽ��� | �����ѡ��״����ܣ���ˮ�� | ��ˮ���� |
| �е�/�� | 68 | 64 |
| ��ע | ������������״����γɹ��л����е�54�� | |
��Բ����ƿ�м���44.8g�״���19.1gNa2B4O7•10H2O����ɰ��ʽ��382��Ȼ�����ؼ���ŨH2SO4�����õ�¯������ƿ�е�Һ�壻����һ��ʱ����Ƚ���51��55�����֣��ٽ���55��60�����֣�����������ֺϲ��������Ȼ��ƽ��������ֲ㣬�ϲ�Ϊ���������������룻����øߴ�����������19.2g��
�ش��������⣺
��1��ͼ������8����������ƿ��ֱ����������ȴˮӦ��a������ĸ���ӿڽ��룮
��2��װ��P2O3�ĸ���������Ƿ�ֹ������ˮ�������뵼������������ˮ�⣮
��3�����ýϸߵļ״�����ɰ�����ʵ���֮�ȵ�Ŀ���������ɰ��ת���ʣ�
��4����������к����������������ϸߵ��ǵ�1���1����2�����Σ�
��5�����������������������������Ҫ���������Ƿ�Һ©����
��6������ʱӦ�ռ�68�����֣�
��7������ʵ��IJ�����D��
A.42.9% B.46.6% C.64.5% D.92.3%
| A�� | �ڢۢܢݢ� | B�� | �ܢݢߢ� | C�� | �ܢݢ� | D�� | �ۢܢݢߢ� |
| A�� | ʳ���ۻ� | B�� | �Ȼ�������ˮ | C�� | �ɱ����� | D�� | �������ۻ� |
 ij����С���ͬѧ���÷�������ȡ���������壬��֪���������������ҺpH��ϵ��ͼ��ʾ��ʵ���п�ѡ�õ��Լ�����������������2.0mol•L-1NaOH��Һ��2.0mol•L-1����
ij����С���ͬѧ���÷�������ȡ���������壬��֪���������������ҺpH��ϵ��ͼ��ʾ��ʵ���п�ѡ�õ��Լ�����������������2.0mol•L-1NaOH��Һ��2.0mol•L-1����