��Ŀ����
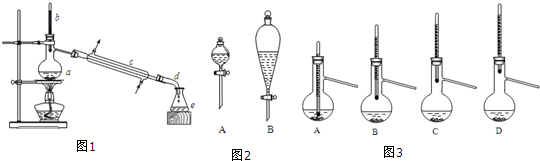
12�� ���������������ܼ�����ˮ�������ϡ����ᡢ����ȵķ�����ȣ�ʵ���Һϳ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
���������������ܼ�����ˮ�������ϡ����ᡢ����ȵķ�����ȣ�ʵ���Һϳ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�Na2B4O7•10H2O+2H2SO4+12CH3OH=2NaHSO4+4��CH3O��3B+17H2O
| ���������� | �״� | |
| ��Է����� | 104 | 32 |
| �ܽ��� | �����ѡ��״����ܣ���ˮ�� | ��ˮ���� |
| �е�/�� | 68 | 64 |
| ��ע | ������������״����γɹ��л����е�54�� | |
��Բ����ƿ�м���44.8g�״���19.1gNa2B4O7•10H2O����ɰ��ʽ��382��Ȼ�����ؼ���ŨH2SO4�����õ�¯������ƿ�е�Һ�壻����һ��ʱ����Ƚ���51��55�����֣��ٽ���55��60�����֣�����������ֺϲ��������Ȼ��ƽ��������ֲ㣬�ϲ�Ϊ���������������룻����øߴ�����������19.2g��
�ش��������⣺
��1��ͼ������8����������ƿ��ֱ����������ȴˮӦ��a������ĸ���ӿڽ��룮
��2��װ��P2O3�ĸ���������Ƿ�ֹ������ˮ�������뵼������������ˮ�⣮
��3�����ýϸߵļ״�����ɰ�����ʵ���֮�ȵ�Ŀ���������ɰ��ת���ʣ�
��4����������к����������������ϸߵ��ǵ�1���1����2�����Σ�
��5�����������������������������Ҫ���������Ƿ�Һ©����
��6������ʱӦ�ռ�68�����֣�
��7������ʵ��IJ�����D��
A.42.9% B.46.6% C.64.5% D.92.3%
���� ��1��������������״����������ˮ�������������෴ʱ������Ч���ã�
��2���ɱ�����Ϣ��֪������������ˮ�⣻
��3������һ�ַ�Ӧ����������������һ�ַ�Ӧ���ת���ʣ�
��4�������������������״��Լ����߹��л����ķе������
��5�����뻥�����ܵ�Һ���÷�Һ©����
��6����������������������
��7��44.8g�״���19.1gNa2B4O7•10H2O����ɰ��ʽ��382����Ӧ���״�������������ɰ�������������������ɵ��������������������ٸ���ʵ�����ɵ�����������������������ʣ�
��� �⣺��1��������������״������֪����8Ϊ��ƿ������ˮ�������������෴ʱ������Ч���ã�������ˮ���¿ڽ��룬���Ͽ����̣�����a���룬
�ʴ�Ϊ����ƿ��a��
��2���ɱ�����Ϣ��֪������������ˮ�⣬����Ҫ��ֹ�����е�ˮ����������ƿ��������������Ӧ����װ��P2O3�ĸ�������÷�ֹ������ˮ�������뵼������������ˮ�⣻
�ʴ�Ϊ����ֹ������ˮ�������뵼������������ˮ�⣻
��3������һ�ַ�Ӧ����������������һ�ַ�Ӧ���ת���ʣ����Բ��ýϸߵļ״�����ɰ�����ʵ���֮�ȣ�������������ɰ��ת���ʣ�
�ʴ�Ϊ�������ɰ��ת���ʣ�
��4���ɱ������ݿ�֪�������������ķе�Ϊ68�棬�״��ķе�Ϊ64�棬������������״��γɵĹ��л����ķе�Ϊ54�棬���Լ������Ȼӷ���������������������״��γɵĹ��л�����51��55�����������������������ϸߣ��ʴ�Ϊ��1��
��5������������ֺϲ��������Ȼ��ƽ��������ֲ㣬�ϲ�Ϊ���������������뻥�����ܵ��ֲܷ��Һ���÷�Һ©�����ʴ�Ϊ����Һ©����
��6���ɱ������ݿ�֪�������������ķе�Ϊ68�棬Ҫ��ô��������������������¶�Ӧ��Ϊ68�棬�ʴ�Ϊ��68��
��7��44.8g�״���19.1gNa2B4O7•10H2O����ɰ��ʽ��382����Ӧ���״�������������ɰ���������㣬����������������������Ϊxg��
Na2B4O7•10H2O+2H2SO4+12CH3OH=2NaHSO4+4��CH3O��3B+17H2O
382 4��104
19.1g xg
��x=$\frac{4��104��19.1g}{382}$=20.8g
���������������IJ���=$\frac{19.2g}{20.8g}$��100%=92.3%��
�ʴ�Ϊ��D��
���� �������������������Ʊ�Ϊ���壬����ѧ�����Ʊ����̵����⡢��ʵ����������⡢��ѧʽ�йؼ���ȣ��Ѷ��еȣ��ۺ��Խ�ǿ���漰��֪ʶ��࣬��Ҫѧ���߱��Ķ������������������֪ʶ�Ľ������������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ���� | B�� | ��ˮ | C�� | Һ�� | D�� | �Ȼ�� |
| ������ | ������ | ���������� | ������ | |
| �ܶ�/��g/cm3�� | 0.810 | 1.049 | 0.882 | 0.7689 |
| �е�/�� | 118.0 | 118.1 | 126.1 | 142 |
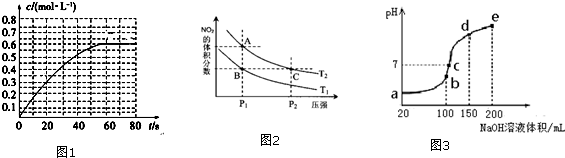
�����ᴿ���ٽ�����a�з�Ӧ��Ļ��Һ������e�е������ϲ���ת���Һ©���У���������ˮ��������10%̼������Һ��ˮϴ�ӣ�������ټӸ����M������һ��ʱ�����ȥM���۽����յõ��ķ�Ӧ�ֲ���ת��ϴ��������a�У����뼸����ʯ���������õ�����������7.31g��
��ش��������⣺
��1������a��������������ƿ��
��2���ڷ�ҺʱӦѡ��ͼ2װ���е�B������ţ���ʹ�ø�����ǰӦ�ȼ�©��
��3��д���Ʊ������������Ļ�ѧ����ʽ��CH3COOH+CH3CH2CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O��
��4����һ��ˮϴ��Ŀ���dz�ȥ���ἰ�������ᣮ
��5�������M������B������ţ�
A������������ B����ˮ������ C����ʯ�� D���������ƹ���
��6������۵õ��Ĵֲ�Ʒ�����ᴿʱ��ͼ3��ʾװ���У��¶ȼ�λ�ÿ��ܵ����ռ����IJ�Ʒ�л��и߷е����ʵ�װ��ΪD������ţ�
��7����ʵ�����õ��������������IJ�����C������ţ�
A��30% B��40% C��50% D��55%
| A�� | 14Cԭ����C60����̼ͨԭ�ӵĻ�ѧ������ͬ | |
| B�� | 14Cԭ����14Nԭ��������������ͬ | |
| C�� | 14C��C60��ͬ�������� | |
| D�� | 14CO��12CO��13CO��̼Ԫ�ص�����ͬλ�� |
| A�� | �÷�Ӧ��NH4NO3ֻ�������� | |
| B�� | ZnO�ǻ�ԭ���� | |
| C�� | ��������ֻ��N2 | |
| D�� | �÷�Ӧÿ����1mol N2ת��5mol���� |

 ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�������������Ԫ��֮һ��Y�Ļ�̬ԭ�����������������ڲ����������2����Z�Ļ�̬ԭ��2p�������3��δ�ɶԵ��ӣ�W��ԭ������Ϊ29��
ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�������������Ԫ��֮һ��Y�Ļ�̬ԭ�����������������ڲ����������2����Z�Ļ�̬ԭ��2p�������3��δ�ɶԵ��ӣ�W��ԭ������Ϊ29��