��Ŀ����
14��ͨ��ʯ���ѽ�ɻ����ϩ��������ϩΪԭ�ϻ��ɺϳɺܶ�Ļ�����Ʒ����֪D����ζ���Ը�����ͼ�ش��й����⣺
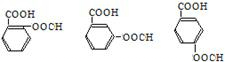
��1���л��� B ������Ϊ��ȩ��A��C �Ĺ��������Ʒֱ����ǻ� ���Ȼ�������ϩ�Ľṹ��ʽΪ
 ��
����2��д��ͼʾ�Т١��ڡ��۷�Ӧ�Ļ�ѧ����ʽ����ָ����Ӧ���ͣ�
��CH2=CH2+H2O$\stackrel{һ��������}{��}$CH3CH2OH����Ӧ���ͣ��ӳɷ�Ӧ
��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O����Ӧ���ͣ�������Ӧ
��C2H5OH+CH3COOH
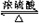 CH3COOC2H5+H2O����Ӧ���ͣ�������Ӧ��
CH3COOC2H5+H2O����Ӧ���ͣ�������Ӧ��
���� C2H4�����Ӿ۷�Ӧ�õ�����ϩ����ϩ��ˮ�����ӳɷ�Ӧ����AΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��BΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��CΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����CH3COOCH2CH3����DΪCH3COOCH2CH3���ݴ˷������
��� �⣺C2H4�����Ӿ۷�Ӧ�õ�����ϩ����ϩ��ˮ�����ӳɷ�Ӧ����AΪCH3CH2OH���Ҵ���Cu��Ag�����������·���������ӦCH3CHO��BΪCH3CHO��CH3CHO�ɽ�һ��������CH3COOH��CΪCH3COOH��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ����CH3COOCH2CH3����DΪCH3COOCH2CH3��
��1��BΪCH3CHO��B ������Ϊ��ȩ��AΪCH3CH2OH��CΪCH3COOH��A�еĹ�����Ϊ�ǻ���C�й�����Ϊ�Ȼ�������ϩ�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ����ȩ���ǻ����Ȼ��� ��
��
��2��ͨ�����Ϸ���֪���٢ڢ۷ֱ��Ǽӳɷ�Ӧ��������Ӧ��ȡ����Ӧ���Ӿ۷�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ���Ӿ۷�Ӧ��
��3������ϩ��ˮ�ӳɷ�Ӧ�����Ҵ�����Ӧ�ķ���ʽΪCH2=CH2+H2O$\stackrel{һ��������}{��}$CH3CH2OH����Ӧ����Ϊ�ӳɷ�Ӧ��
�ڷ�ӦΪ�Ҵ��Ĵ���������Ӧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O����Ӧ����Ϊ������Ӧ��
�����Ҵ��������������Ӧ����Ӧ����ʽΪC2H5OH+CH3COOH  CH3COOC2H5+H2O����Ӧ����Ϊ������Ӧ��
CH3COOC2H5+H2O����Ӧ����Ϊ������Ӧ��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{һ��������}{��}$CH3CH2OH���ӳɷ�Ӧ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��������Ӧ��C2H5OH+CH3COOH 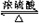 CH3COOC2H5+H2O��������Ӧ��
CH3COOC2H5+H2O��������Ӧ��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ��������漰ϩ������ȩ�����ᡢ��֮���ת����ע���Ϸ�Ӧ���������ƶϣ�֪���Ҵ��Ĵ������ϼ���ʽ����Ŀ�ѶȲ���

��1��2CH3CHO$��_{��}^{NaOH}$CH3CH��OH��CH2CHO$��_{��}^{-H_{2}O}$CH3CH=CHCH0$\stackrel{��}{��}$CH3CH2CH2CH0
��2��

��3�������ڱ�������������2-�һ�-1-�����ϳ�DEHP

����˵����ȷ���ǣ�������
| A�� | �ϳɹ����漰�ӳɡ���ȥ���������ۺϵ����͵��л���Ӧ | |
| B�� | ���裨1���Тڷ�Ӧ��������������NaOH/����Һ�м��ȣ���Ӧ��������ȥ��Ӧ | |
| C�� | ���裨1����2����������ʱ���ۡ�������H2���ʵ������ | |
| D�� | ��Ӧ����ʽ�в���H2Oϵ��x=2����ʱ��ȥ���з�Ӧ���ڵ�ˮ�����DEHP���� |
| A�� | 1 | B�� | 12 | C�� | 16 | D�� | 23 |
| ȼ�� | һ����̼ | ���� | �����飨C8H18�� | �Ҵ� |
| ��H | -283.0kJ•mol-1 | -891.0kJ•mol-1 | -5461.0kJ•mol-1 | -1366.8kJ•mol-1 |
| A�� | һ����̼ | B�� | ���� | C�� | ������ | D�� | �Ҵ� |
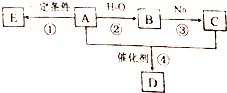 ����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28��C��һ�����壬E��һ�ָ߷��ӻ����A��B��C��D��E��һ�������´�����ͼת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ�
����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28��C��һ�����壬E��һ�ָ߷��ӻ����A��B��C��D��E��һ�������´�����ͼת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ� ����֪һ��̼ԭ�������������ǻ�ʱ����������ת����
����֪һ��̼ԭ�������������ǻ�ʱ����������ת����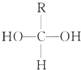 $\stackrel{-H_{2}O}{��}$
$\stackrel{-H_{2}O}{��}$
 ��
��
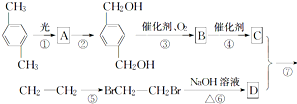 E��C12H14O6��
E��C12H14O6��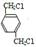 ��B
��B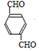 D
D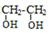 ��
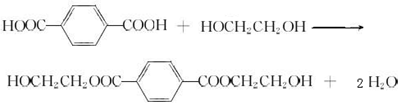
��
 ��
��