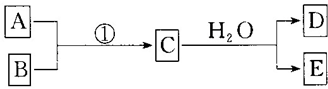
��Ŀ����
17������˵��������ǣ�������| A�� | ��1L1mol/L���Ȼ�����Һ��ȡ��10ml����Ũ������1mol/L | |
| B�� | ��80 g NaOH����1 Lˮ�У�������Һ��NaOH�����ʵ���Ũ��Ϊ2 mol/L | |
| C�� | 0.5 L 2mol/L���Ȼ�����Һ�У������Ӻ�����������ԼΪ3��6.02��1023 | |
| D�� | 10g 98%���ᣨ�ܶ�Ϊ1.84g/cm3����10mL18.4mol/L��������ʵ���Ũ������ͬ�� |
���� A����Һ���о�һ�ԣ���Һ���ʵ���Ũ������Һ����أ�
B.80gNaOH�����ʵ�����2mol����80 g NaOH����1 Lˮ�У���Һ�������1L��
C���Ȼ������ʵ���=2mol/L��0.5L=1mol�����ݻ�ѧʽ֪�������Ӻ������ӵ����ʵ���֮��Ϊ3mol������N=nNA������������
D.98%���ܶ�Ϊ1.84g/cm3��Ũ�������ʵ���Ũ��=$\frac{1000�Ѧ�}{M}$��
��� �⣺A����Һ���о�һ�ԣ���Һ���ʵ���Ũ������Һ����أ����Դ�1L1mol/L���Ȼ�����Һ��ȡ��10ml����Ũ������1mol/L����A��ȷ��
B.80gNaOH�����ʵ�����2mol����80 g NaOH����1 Lˮ�У���Һ�������1L�������Һ���ʵ���Ũ��С��2mol/L����B����
C���Ȼ������ʵ���=2mol/L��0.5L=1mol�����ݻ�ѧʽ֪�������Ӻ������ӵ����ʵ���֮��Ϊ3mol������N=nNA�ñ����Ӻ�����������ԼΪ3��6.02��1023����C��ȷ��
D.98%���ܶ�Ϊ1.84g/cm3��Ũ�������ʵ���Ũ��=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L�����Զ������ʵ���Ũ����ȣ���D��ȷ��
��ѡB��
���� ���⿼�����ʵ����йؼ��㣬Ϊ��Ƶ���㣬��ȷ���ʵ�����ʽ�и���������֮���ϵ�ǽⱾ��ؼ����״�ѡ����B��ע�����ʵ���Ũ�ȹ�ʽ��Vָ��Һ����������ܼ������Ϊ�״��㣮

| A�� | ͬ��������Ԫ�أ�ԭ�Ӱ뾶��ԭ��������������� | |
| B�� | ��3��������Ԫ�ص���������ϼ۵������������������� | |
| C�� | ���ڱ�������Ԫ�������������Ǣ�A�� | |
| D�� | ϡ������Ԫ��ԭ�ӵ�������������Ϊ8 |
| A�� | ��ԭ�� | B�� | ������ | C�� | ��ԭ���� | D�� | �������� |
| A�� | ��˾ƥ�ֿν�����ʹ�����������Ƹ�ð | |
| B�� | �����������к�θ�ᣬ����������θ����� | |
| C�� | ��˿����Ҫ�ɷ�����ά�� | |
| D�� | �Ͻ���һ��ֻ������Ԫ�� |
�ٿ��� ����������������������Ȼ�� ������ˮú�������ݿ��� ��ʯ��Һ������
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ڢܢ� | D�� | �٢ܢ� |
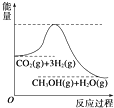 ��1����˹���ɵĺ����ǣ�����һ����ѧ��Ӧ��������һ����ɻ��Ƿּ�����ɣ��䷴Ӧ�ʱ䶼����ͬ�ģ����仰˵����ѧ��Ӧ�ķ�Ӧ��ֻ����ϵ��ʼ̬����̬�йأ����뷴Ӧ��;����
��1����˹���ɵĺ����ǣ�����һ����ѧ��Ӧ��������һ����ɻ��Ƿּ�����ɣ��䷴Ӧ�ʱ䶼����ͬ�ģ����仰˵����ѧ��Ӧ�ķ�Ӧ��ֻ����ϵ��ʼ̬����̬�йأ����뷴Ӧ��;����