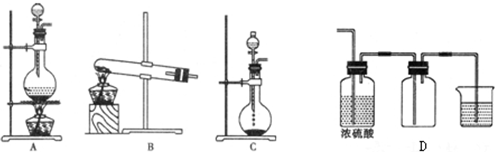
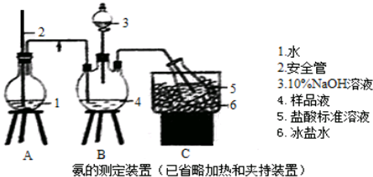
��Ŀ����
20�� �屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽���������뱽������ȡ����Ӧ��װ��ʾ��ͼ���й��������£�
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽���������뱽������ȡ����Ӧ��װ��ʾ��ͼ���й��������£��ش��������⣺
��1������d���������������ܣ�
��2����a�м���15mL����������м��b�м���4.0mLҺ̬�壮Ϊ����֤�����巢����ȡ����Ӧ����B��ƿ��Ӧ������Լ�������AgNO3��Һ����ʯ����Һ����ͨ��b��a�е���Һ��ʱ��B��ƿ���а���������������Ϊ������HBr���壻ͬʱ���ɹ۲쵽��dz��ɫ�������ɣ�����Һ��죩������CCl4�����ó�ȥHBr�л��е���������
��3��Һ�����������в�������ᴿ��
��ͨ��c��a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м����ˮ�������ܽ�HBr��
����Һ������10mLˮ��8mL10%��NaOH��Һ��10mLˮϴ�Ӻ��÷�Һ���ڶ�����ˮϴ�ӵ�������ϴȥ������NaOH�����ɵ��Σ���ҺʱB������ţ���
A��ֱ�ӽ��屽�ӷ�Һ©���Ͽڵ���
B��ֱ�ӽ��屽�ӷ�Һ©���¿ڷų�
C���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��屽���¿ڷų�
D���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��屽���Ͽڵ���
��4�������Ϸ��������ͨ�������һ���ᴿ���屽�Ĺ����в���Ҫ�õ���������CD������ȷ�𰸱�ţ���
A��������ƿ��������B���¶ȼơ�����C������ƿ������D������������ E��������
��5�����屽�������ᴿ��ô��屽Ϊ3.9g�����屽������32%��ȡ��������
| ��Ŀ | �� | �� | �屽 |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/��C | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
���� ��1������d�Ĺ����֪��dΪ���������ܣ�
��2��֤������ȡ����Ӧ��ֻҪ֤����Һ�����廯�����ɼ��ɣ���������������Һ��ʯ����Һ���飻����Һ�巴Ӧ����HBr��HBr��ˮ������ϳʰ��������������л��ܼ��������Ȼ�̼���Գ�ȥ�廯���л��е��嵥�ʣ�
��3����a�������ɵ��廯�⣬�廯��������ˮ���������л��ܼ�������ˮ�����ܽ��廯�⣻
������������ϴȥ�ܽ������ᣬˮϴ��ȥδ��Ӧ��NaOH�����ɵ��Σ����ɵ��屽�ܶȴ���ˮ�����Һ����ʱ��ֱ�Ӵӷ�Һ©���¶˷ų����ɣ�
��4������������������ж���Ҫʹ�õ�������
��5���ֱ���ݷ�Ӧ���������ܶȼ�����������ټ��������������ʵ�����Ȼ���жϹ�����������ݲ���������������������屽�����ʵ������ٸ���m=nM������屽����������������屽�IJ��ʣ�
��� �⣺��1��dΪ���������ܣ������������ã�
�ʴ�Ϊ�����������ܣ�
��2��Ϊ����֤�����巢����ȡ����Ӧ����������ƿ�м���AgNO3��Һ����ʯ����Һ��������dz��ɫ�������ɣ�����Һ��죩����˵����Ӧ�����廯�����ɣ�������ȡ����Ӧ����Ӧ���ɵ��廯������ˮ��������ְ����������������Ȼ�̼�������Ȼ�̼��Һ�ɳ�ȥHBr�л��е���������
�ʴ�Ϊ��AgNO3��Һ����ʯ����Һ����HBr����dz��ɫ�������ɣ�����Һ��죩����ȥHBr�л��е���������
��3����ͨ��c��a�м���10mLˮ��Ŀ�����ܽ�a�����ɵ��廯�⣬
�ʴ�Ϊ���ܽ�HBr��
������ˮϴ��ȥŨ���ᡢ���ᣬ�����������Ƴ�ȥ�ܽ�������ᣬ���ˮϴ��ȥδ��Ӧ��NaOH�����ɵ��Σ��屽���ܶȴ���ˮ��Һ�����Һ����ʱֱ�ӽ��屽�ӷ�Һ©���¿ڷų�������B��ȷ��
�ʴ�Ϊ��ϴȥ������NaOH�����ɵ��Σ�B��
��4��ͨ�������һ���ᴿ���屽�Ĺ����У���Ҫʹ���¶ȼơ�������ƿ��������������Ҫʹ�������ܺ�����ƿ������CD��ȷ��
�ʴ�Ϊ��CD��
��5��15mL��������Ϊ��0.88g/mL��15=13.2g�������ʵ���Ϊ��$\frac{13.2g}{78g/mol}$=0.169mol��
4.0mLҺ�������Ϊ��3.10g/mL��4.0mL=12.4g�������ʵ���Ϊ��$\frac{12.4g}{160g/mol}$=0.0775mol��
��Ȼ�岻�㣬�����������屽�����ʵ���Ϊ��0.0775mol�������屽������Ϊ��157g/mol��0.0775mol��12.17g��
�屽�IJ���Ϊ��$\frac{3.9g}{12.17g}$��100%=32%��
�ʴ�Ϊ��32%��
���� ���⿼���������Ʊ���������ƣ���Ŀ�Ѷ��еȣ��漰�������ơ���ѧʵ�����������������ѧ�����֪ʶ������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���Ļ�ѧʵ�顢��ѧ����������

| A�� | HClO4��H2SO4��H3PO4������������ǿ | |
| B�� | P��S��Cl������������ | |
| C�� | HCl��HBr��HI���ȶ���������ǿ | |
| D�� | �ơ�þ�����Ľ��������μ��� |
| A�� | HCl��ˮ | B�� | CO2��NaOH��Һ | C�� | NO��ˮ | D�� | Cl2��NaOH��Һ |
| A�� | c��OH-��=c��H+��+c��HS-��+c��H2S�� | |
| B�� | ��Һ�и�����Ũ���ɴ�С��˳��Ϊ��c��Na+����c��OH-����c��HS-����c��S2-����c��H+�� | |
| C�� | 2 c��Na+��=c��S2-��+c��HS-��+c��H2S�� | |
| D�� | ����0.1mol•L-1��Na2S��Һʱ���������KOH����S2-��ˮ�� |
| A�� | ���Ȼ�̼���ӱ���ģ�ͣ� | B�� | COS�ĵ���ʽ�ǣ�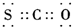 | ||
| C�� | ������ĽṹʽΪH-Cl-O | D�� | ${\;}_{8}^{18}$O2-���ӽṹʾ��ͼ��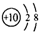 |
| A�� | ԭ��������������X��Y��Z | B�� | ���Ӱ뾶��r��W3+����r��Y2-����r��Z+�� | ||
| C�� | MY2��Z2Y�Ļ�����������ͬ | D�� | ���ʷе㣺M��Z��Y |
| A�� | ��֬����֬�� | B�� | ��֬������ʹ��ˮ��ɫ | ||
| C�� | ��֬û�й̶����۷е� | D�� | ��֬�Ǹ�֬����ĸ����� |