��Ŀ����
����Ŀ���������⣩
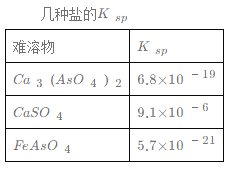
(һ)��������ѧ���о�����������ϩ������Ϊԭ�ϡ��Ӷ����������ϳ������������¹��գ����������Ҵ�����ȩ���м��壬ʹ��Ʒ�ɱ����ͣ��������Ծ������ơ���ϳɵĻ�����Ӧ���£�
CH2=CH2(g)+CH3COOH(1)![]() CH3COOC2H5(1)
CH3COOC2H5(1)
(1)����������˵���̶���������ϩ������ϳ����������ķ�Ӧ�Ѵﻯѧƽ�����___________��
A�����ᡢ����������Ũ����ͬ
B�������ϳɷ�Ӧ�����������ֽⷴӦ���������
C����ϵ�������ܶ�һ��
D����ϩ�Ͽ�1mol̼̼˫����ͬʱ����ǡ������1mol
(2)��n(��ϩ)��n(����)���ϱ�Ϊ1�������£�ij�о�С���ڲ�ͬѹǿ�½���������ͬʱ������������IJ������¶ȵı仯�IJⶨʵ�飬ʵ������ͼ��ʾ���ش��������⣺
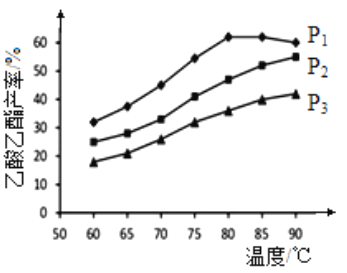
���¶���60��90�淶Χ�ڣ���ϩ�����������ϳɷ�Ӧ�����ɴ�С��˳����___________[��v(P1)��v(P2)��v(P3)�ֱ��ʾ��ͬѹǿ�µķ�Ӧ����]��������ԭ��Ϊ______________________��
����ѹǿΪP1MPa���¶ȳ���80��ʱ���������������½���ԭ�������______________________��
�۸��ݲⶨʵ���������������˵�����������______________________(������ʵ�ѹǿ���¶�)��Ϊ������������IJ����ʹ��ȣ����Բ�ȡ�Ĵ�ʩ��______________________(��д��һ��)��
(3)��֪��Ӧ�۵ı�ƽ�ⳣ��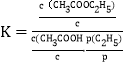 ������c��Ϊ��Ũ��(1.0mol/L)��p(C2H4)Ϊƽ��ϵͳ��C2H4��ƽ���ѹ��p(C2H4)=p��x(C2H4)��x(C2H4)Ϊƽ��ϵͳ��C2H4�����������p��Ϊ��ѹǿ(1.0��105Pa)���������ʵ�������ϩ��������80���10MPa�·�Ӧ������������ƽ�����Ϊ80%����K��=__________��
������c��Ϊ��Ũ��(1.0mol/L)��p(C2H4)Ϊƽ��ϵͳ��C2H4��ƽ���ѹ��p(C2H4)=p��x(C2H4)��x(C2H4)Ϊƽ��ϵͳ��C2H4�����������p��Ϊ��ѹǿ(1.0��105Pa)���������ʵ�������ϩ��������80���10MPa�·�Ӧ������������ƽ�����Ϊ80%����K��=__________��
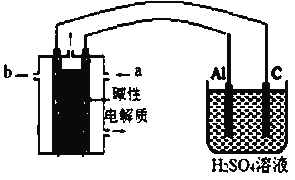
(��)ij��ѧ��ȤС����ʵ������ģ������Ʒ�������ۻ�����������(װ����ͼ��ʾ)���ɹ�ѡ����Լ��У��״���������KOH��Һ����������b��__________�����缫�ĵ缫��ӦΪ__________��
���𰸡�BC v(P1)��v(P2)��v(P3) ����������ͬʱ�������������ķ�Ӧ��ѹǿԽ��ѧ��Ӧ����Խ�� ��ͼ���֪��P1MPa��80��ʱ��Ӧ�Ѵ�ƽ��������Ӧ���ȣ���ѹǿ���������¶�ƽ�������ƶ������½� P1MPa��80�� ͨ����ϩ���������ѹǿ 0.04 �״�(��CH3OH) Al+3H2O-6e-=Al2O3+6H+
��������
��һ������1��A.���ᡢ����������Ũ����ͬ����һ�����䣬��һ���ﵽƽ��״̬����A����
B.�����ϳɷ�Ӧ�����������ֽⷴӦ��������ȣ�˵����Ӧ�ﵽƽ��״̬����B��ȷ��
C.��ϵ�������ܶ�һ����˵�����淴Ӧ������ȣ���Ӧ�ﵽƽ��״̬����C��ȷ��
D.��ϩ�Ͽ�1mol̼̼˫����ͬʱ����ǡ������1mol����ʾ�淴Ӧ������ȣ���Ӧ��һ���ﵽƽ��״̬����D����
�ʴ�Ϊ��BC��
��2��������Ӧ�������С�ģ�����ѹǿ���������IJ�����������������ͬʱ�������������ķ�Ӧ��ѹǿԽ��ѧ��Ӧ����Խ�죬�����¶���60��80�淶Χ�ڣ���ϩ�����������ϳɷ�Ӧ�����ɴ�С��˳����![]() (P1)��
(P1)��![]() (P2)��
(P2)��![]() (P3)���ʴ�Ϊ��v(P1)��v(P2)��v(P3)������������ͬʱ�������������ķ�Ӧ��ѹǿԽ��ѧ��Ӧ����Խ�졣
(P3)���ʴ�Ϊ��v(P1)��v(P2)��v(P3)������������ͬʱ�������������ķ�Ӧ��ѹǿԽ��ѧ��Ӧ����Խ�졣
����ͼ���֪��P1MPa��80��ʱ��Ӧ�Ѵ�ƽ��������Ӧ���ȣ���ѹǿ���������¶�ƽ�������ƶ������½����ʴ�Ϊ����ͼ���֪��P1MPa��80��ʱ��Ӧ�Ѵ�ƽ��������Ӧ���ȣ���ѹǿ���������¶�ƽ�������ƶ������½���
�۸��ݲⶨʵ���������������˵�����������P1MPa��80�档����Ӧ�����С��Ϊ������������ĺϳ����ʺͲ��ʣ����Բ�ȡ�Ĵ�ʩ��ͨ����ϩ���������ѹǿ���ʴ�Ϊ��P1MPa��80�棻ͨ����ϩ���������ѹǿ��
��3���跴Ӧ�����ʼ��ԼΪ1mol����
CH2=CH2(g)+CH3COOH(1)![]() CH3COOC2H5(1)
CH3COOC2H5(1)
��ʼ��mol�� 1 1 0
�仯��mol�� 0.8 0.8 0.8
ƽ�⣨mol�� 0.2 0.2 0.8
��Ӧǰ�������ѹǿ���䣬��ͬ�����У���ͬҺ���Ũ��֮�ȵ��������ʵ���֮�ȣ���K��=c(CH3COOC2H5)/c��/[c(CH3COOH)/c����p(C2H4)/p��]=n(CH3COOC2H5)p��/n(CH3COOH) p(C2H4)=0.8mol��1.0��105Pa/0.2mol��1.0��106Pa=0.04���ʴ�Ϊ��0.04��
������������ʯī��������Al���������������ҺΪ������Һ�������缫�ĵ缫��ӦΪAl+3H2O-6e-=Al2O3+6H+����������������Ĥ����ȼ�ϵ�صĸ����������ǵ��ص������������ϼ״�ʧȥ���ӷ���������Ӧ��������bΪ�״����ʴ�Ϊ���״�(��CH3OH)��Al+3H2O-6e-=Al2O3+6H+��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�