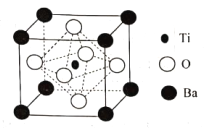
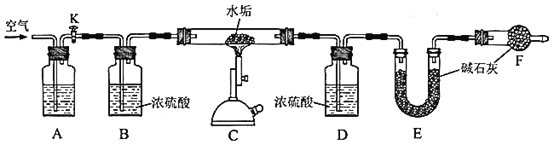
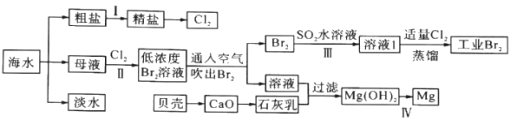
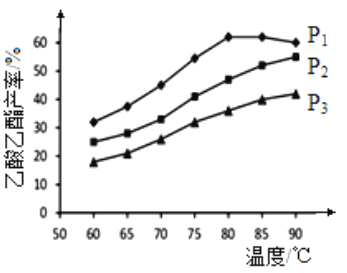
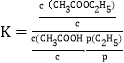��Ŀ����
����Ŀ���Թ�ҵ��ˮ��������ˮ���д����Ƿ�ֹˮ����Ⱦ������ˮ�ʵ���Ҫ��ʩ֮һ�����᳧�����Է�ˮ����(As)Ԫ��(��Ҫ��H3AsO3��ʽ����)�������ߣ�Ϊ��������ŷţ�ij�������û�ѧ���������������ˮ����ش��������⣺
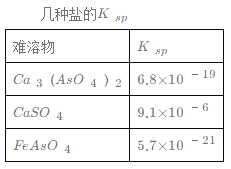
��1�������Է�ˮ��Fe3+��Ũ��Ϊ1.0��10-4 mol��L-1����c(AsO43-)������ ____mol��L-1��
��2�������ŷų������Է�ˮ�е�������(����H3AsO3)���׳�������Ͷ��MnO2�Ƚ�������������� (����H3AsO4)����ʱMnO2����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ_________________��
��3������(H3AsO4)�ֲ������ƽ�ⳣ��(25 ��)ΪKa1=5.6��10-3��Ka2=1.7��10-7��Ka3=4.0��10-12�������������ƽ�ⳣ������ʽΪKa3=_________��Na3AsO4��һ��ˮ������ӷ���ʽΪAsO43��+H2O![]() HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��(25 ��)Ϊ____��
HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��(25 ��)Ϊ____��
���𰸡�5.7��10-17 2H++MnO2+H3AsO3![]() H3AsO4+Mn2++H2O
H3AsO4+Mn2++H2O ![]() 2.5��10-3
2.5��10-3
��������
������Ҫ�������ܵ���ʵ��ܽ�ƽ�⼰����ת���ı��ʡ�
��1�������ܶȻ���������õ�AsO43-��Ũ�ȣ�
��2�����ݷ�Ӧ����������ϵ�����Ԫ�ػ��ϼ۵ı仯��ƽ����ʽ��
��3��H3AsO4�ĵ���������ʽΪHAsO42-![]() H++AsO43-������д�������ƽ�ⳣ���ı���ʽ������ˮ�ⳣ������볣����Kw�Ĺ�ϵ������
H++AsO43-������д�������ƽ�ⳣ���ı���ʽ������ˮ�ⳣ������볣����Kw�Ĺ�ϵ������
��1������Ksp(FeAsO4)=c(Fe3+)��c(AsO43-)=5.7��1021��Fe3+��Ũ��Ϊ1.0��104molL1����c(AsO43-)=Ksp(FeAsO4)/c(Fe3+)=5.7��1017mol/L��
��2��������(H3AsO3����)���׳�������Ͷ��MnO2�Ƚ��������������(H3AsO4����)����÷�Ӧ�����ӷ���ʽΪ��2H++MnO2+H3AsO3===H3AsO4+Mn2++H2O��
��3��H3AsO4�ĵ���������ʽΪHAsO42��![]() H++ AsO43�������Ե����������ƽ�ⳣ���ı���ʽΪ
H++ AsO43�������Ե����������ƽ�ⳣ���ı���ʽΪ![]() ��Na3AsO4�ĵ�һ��ˮ������ӷ���ʽΪ��AsO43��+H2O
��Na3AsO4�ĵ�һ��ˮ������ӷ���ʽΪ��AsO43��+H2O![]() HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��
HAsO42��+OH-���ò�ˮ���ƽ�ⳣ��![]() ��
��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����Ŀ��ijС��ͬѧ̽�����ʵ��ܽ�ȴ�С�����ת������֮��Ĺ�ϵ����֪��
���� | BaSO4 | BaCO3 | AgI | AgCl | |
�ܽ��/g��20�棩 | 2.4��10-4 | 1.4��10-3 | 3.0��10-7 | 1.5��10-4 |
��1��̽��BaCO3��BaSO4֮���ת��
ʵ�������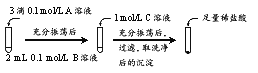
�Լ�A | �Լ�B | �Լ�C | �������������� | |
ʵ��� | BaCl2 | Na2CO3 | Na2SO4 | ���� |
ʵ��� | Na2SO4 | Na2CO3 | ���������ݲ��������������ܽ� |
�� ʵ���˵��BaCO3ȫ��ת��ΪBaSO4�����ݵ������Ǽ��������______��
�� ʵ����м���ϡ���������Ӧ�����ӷ���ʽ��______��
�� ʵ���˵�����������˲���ת�������BaSO4�ij����ܽ�ƽ�����ԭ��______��
��2��̽��AgCl��AgI֮���ת��
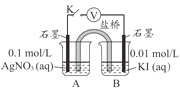
ʵ���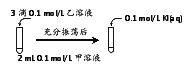
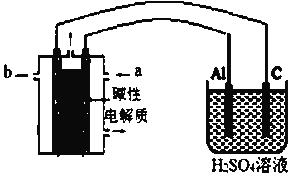
ʵ��������Թ��н�����Һ�䷴Ӧʱ��ͬѧ�����۲쵽AgIת��ΪAgCl�����������������ʵ�飨��ѹ��������a��c��b��0����
װ�� | ���� | ��ѹ������ | |
| ��.��ͼ����װ�ò������Լ����պ�K | a | |
��.��B�е���AgNO3(aq)����������ȫ | b | ||
��.����B��Ͷ��һ����NaCl (s) | c | ||
��.�ظ���������B�м����뢣����NaCl(s) | a |
ע��������������ʱ������ԭ��ط�Ӧ������������ԭ�����������ԣ���ԭ�ԣ�Խǿ��ԭ��صĵ�ѹԽ�����ӵ������ԣ���ԭ�ԣ�ǿ������Ũ���йء�
�� ʵ���֤����AgClת��ΪAgI������Һ������______������ţ���
a. AgNO3��Һ b. NaCl��Һ c. KI��Һ
�� ʵ����IJ��袡�У�B��ʯī�ϵĵ缫��Ӧʽ��______��
�� �����Ϣ������ʵ�����b��a��ԭ��______��
�� ʵ�����������˵��AgIת��ΪAgCl��������______��
��3���ۺ�ʵ������ɵó����ۣ� ______��