��Ŀ����
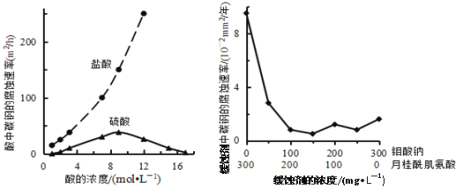
����Ŀ����ij�¶��£������ᣨ�����ᣩ��ˮϡ�����У���Һ�ĵ���������ͼ��ʾ����

��1����ʪ���pH��ֽ����a����Һ��pH���������______��ƫ��ƫС�䣩
��2���Ƚ�a��b��c������������ݴ�С�������ϵ������a��b��c��c=a��b��a=b=c�ȣ�
�ٴ���ĵ���̶ȣ�_________��
��a��b��c������Һ��1mol/L����������Һ�кͣ���������������Һ�����______��
��3����ϡ�����У�����c��CH3COOH���Ľ��ͣ�����ʼ�ձ����������Ƶ�����______��
A��c��H+�� B��H+���� C��CH3COOH���� D��![]()
���𰸡�ƫС c��a��b a=b=c BD
��������
������Ҫ��������������֪ʶ����1����ʪ���pH��ֽ����a����Һ��pH������ڽ���Һϡ�ͣ�
��2������ҺԽϡ��Խ�ٽ�������룻��abc�������Ũ�Ȳ�ͬ������������������ӵ����ʵ���֮����ͬ��
��3����ˮϡ�ͣ��ٽ����룬n��CH3COO-����n��H+������n��CH3COOH����С���Դ˽��
��1����ͼ��֪��a������ϡ�ͣ�������Ũ������������ʪ���pH��ֽ����a����Һ��pH���������ƫС��
��2������ҺԽϡ��Խ�ٽ�������룬����ĵ�����ɴ�С��˳��Ϊc>b>a��
��abc�������Ũ�Ȳ�ͬ������������������ӵ����ʵ���֮����ͬ������a��b��c������Һ��1mol/L����������Һ�кͣ���������������Һ�����ͬ��
��3��A.��ϡ�����У���Һ��������������ӵ�Ũ�ȼ�С����A����
B.�������Խϡ�������Խ�����������������Խ�࣬��B��ȷ��
C.�������Խϡ�������Խ��ƽ�������ƶ���CH3COOH���������٣���C����
D.��ˮϡ�ͣ��ٽ����룬n��CH3COO-����n��H+������n��CH3COOH����С����![]() ����D��ȷ��
����D��ȷ��