��Ŀ����
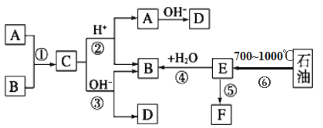
����Ŀ������������Ԫ��X��Z��Y��P��ԭ����������������Ԫ���γɵĻ�����ṹ��ͼ��ʾ�����и�ԭ�ӵĺ�������Ų��������ȶ��ṹ����X��Z��Y��P�����γɵ�������ȵķ��ӣ�X2P���³�ѹ��ΪҺ�塣����˵������ȷ���ǣ� ��
![]()
A.X��P�γɵĻ������п��ܴ��ڷǼ��Թ��ۼ�
B.Z��Y��P�ķǽ����Ժ�����������Ӧˮ��������Զ�����ǿ
C.Y���⻯���ܺ���������������Ӧˮ���ﷴӦ������
D.X�γɵļ����Ӱ뾶��һ����Li+С
���𰸡�B
��������
����������Ԫ��X��Z��Y��P��ԭ����������������Ԫ���γɵĻ�����ṹ��ͼ��ʾ�����и�ԭ�ӵĺ�������Ų��������ȶ��ṹ����X��Z��Y��P�����γɵ�������ȵķ��ӣ�X2P���³�ѹ��ΪҺ�壬���Ƴ�XΪH��ZΪC��YΪN��PΪO��
A. X��P�γɵĻ�����H2O2�д��ڷǼ��Թ��ۼ�����A��ȷ��
B. C��N��O�ķǽ���������ǿ��O������������Ӧˮ�����B����
C. Y���⻯����NH3����������������Ӧˮ����HNO3��Ӧ������NH4NO3����C��ȷ��
D. ����ͬ���Ӳ�ṹ�˶ྶС�����X�γɵļ�����H���뾶���ڱ�Li+����D��ȷ��
������������ΪB��

����Ŀ��ij��ȤС���ö�п��Ƥ�����������Ʊ���ˮ������п(ZnSO4��7H2O)
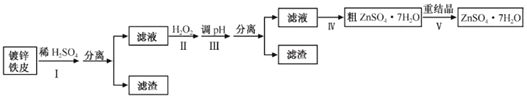
�����Ϣ���£�
�ٽ��������γ�����������������pH��Χ��
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 1.5 | 2.8 |
Fe2+ | 5.5 | 8.3 |
Zn2+ | 5.4 | 8.2 |
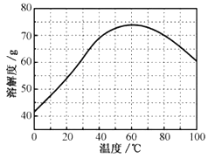
��ZnSO4���ܽ��(������100gˮ���ܽ������)���¶ȱ仯���ߡ���ش�
��1���ٶ�п��Ƥ�ϵ����ۿ���Na2CO3��Һȥ����������__��
�ڲ����������ж϶�п����ȫ��Ӧ��ʵ��������__��
��2���������������H2O2������__��
��3��������ʵ�pH��Χ��__��
��4�����������Ҫ�õ��������в�����a.��������Һ���־�Ĥ��ֹͣ���ȣ�b.��60�������ܼ���c.��ȴ�����£�d.��100�������ܼ���e.���ˡ�
�����������������ȷ˳��__��(�������ظ�ʹ��)
��5���������ijͬѧ���ò�ͬ���·�ʽ������ȴ�ᾧ�����ZnSO47H2O���������С�ֲ���ͼ��ʾ�����ݸ�ʵ������Ϊ�˵õ�������С��Ծ�һ�Ľϴ�������ѡ��__��ʽ������ȴ�ᾧ��
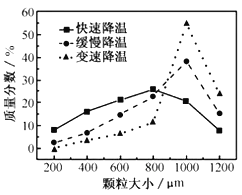
A.���ٽ��� B.�������� C.���ٽ���
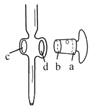
��6����ZnSO47H2O��Ʒ�Ĵ��ȿ�����λ�ζ����ⶨ��
���й��ڵζ���������ȷ����__��
A.ͼ�У�Ӧ����ʿ��Ϳ��������a�˺��������ڵ�c��
B.�ζ�ǰ����ƿ�͵ζ��ܾ����ñ���Һ��ϴ
C.������Һװ��ζ���ʱ��Ӧ�����ձ���©���Ȳ�������ת��
D.�ζ�ʱ��ͨ�������ֿ��������μ���Һ������ҡ����ƿ��ʹ��Һ��ͬһ������ת
E.�ζ�ǰ�ζ��ܼ����������ݣ��ζ�������������ݣ����õ������ʵ�����ĵ�С
��ͼ�еζ��յ��ǵĶ�����___mL��