��Ŀ����
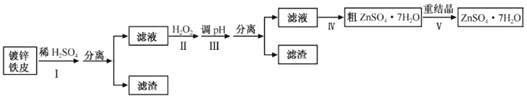
����Ŀ��ij��ȤС���ö�п��Ƥ�����������Ʊ���ˮ������п(ZnSO4��7H2O)
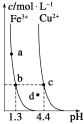
�����Ϣ���£�
�ٽ��������γ�����������������pH��Χ��
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 1.5 | 2.8 |
Fe2+ | 5.5 | 8.3 |
Zn2+ | 5.4 | 8.2 |
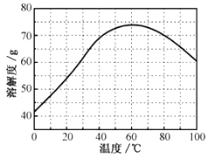
��ZnSO4���ܽ��(������100gˮ���ܽ������)���¶ȱ仯���ߡ���ش�
��1���ٶ�п��Ƥ�ϵ����ۿ���Na2CO3��Һȥ����������__��
�ڲ����������ж϶�п����ȫ��Ӧ��ʵ��������__��
��2���������������H2O2������__��
��3��������ʵ�pH��Χ��__��
��4�����������Ҫ�õ��������в�����a.��������Һ���־�Ĥ��ֹͣ���ȣ�b.��60�������ܼ���c.��ȴ�����£�d.��100�������ܼ���e.���ˡ�
�����������������ȷ˳��__��(�������ظ�ʹ��)
��5���������ijͬѧ���ò�ͬ���·�ʽ������ȴ�ᾧ�����ZnSO47H2O���������С�ֲ���ͼ��ʾ�����ݸ�ʵ������Ϊ�˵õ�������С��Ծ�һ�Ľϴ�������ѡ��__��ʽ������ȴ�ᾧ��
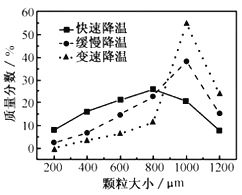
A.���ٽ��� B.�������� C.���ٽ���
��6����ZnSO47H2O��Ʒ�Ĵ��ȿ�����λ�ζ����ⶨ��
���й��ڵζ���������ȷ����__��
A.ͼ�У�Ӧ����ʿ��Ϳ��������a�˺��������ڵ�c��
B.�ζ�ǰ����ƿ�͵ζ��ܾ����ñ���Һ��ϴ
C.������Һװ��ζ���ʱ��Ӧ�����ձ���©���Ȳ�������ת��
D.�ζ�ʱ��ͨ�������ֿ��������μ���Һ������ҡ����ƿ��ʹ��Һ��ͬһ������ת
E.�ζ�ǰ�ζ��ܼ����������ݣ��ζ�������������ݣ����õ������ʵ�����ĵ�С
��ͼ�еζ��յ��ǵĶ�����___mL��
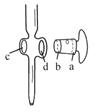

���𰸡�Na2CO3ˮ�⣬��Һ�ʼ��ԣ���ʹ��֬ˮ�� �������ݵ������������� ʹFe2+������ת��ΪFe3+��H2O2�ֽ� 2.8��5.4 dabace C ADE 20.60
��������
��п��Ƥ�Ƚ���������ܽ�������ӣ��ٸ��ݽ������ӳ�������ȥ�����ӣ�����п��Һ�����õ����塣
(1)Na2CO3ˮ�⣬��Һ�ʼ��ԣ���ʹ��֬ˮ�⣬������ϴ�����ۣ���пFeƬ�ܽ���ϡ����ʱ������������������ʽϿ죬���۲쵽�������ݵ�������������ʱ���ж϶�п����ȫ��Ӧ��
�ʴ�Ϊ��Na2CO3ˮ�⣬��Һ�ʼ��ԣ���ʹ��֬ˮ�⣻�������ݵ���������������
(2)˫��ˮ���������ԣ���������ԭ������Fe2+��Ȼ�������Һ��pH���Ӷ���ȥ�����ӣ����ӷ���ʽΪ![]() ��
��
�ʴ�Ϊ��ʹFe2+������ת��ΪFe3+��H2O2�ֽ⣻
(3)�ɱ������ݿ�֪����pH����ʹ��������ȫ���ɳ�����������Ӱ��п���ӣ���pH��ΧΪ2.8��pH��5.4���ʴ�Ϊ��2.8��5.4��
(4)����Һ�����õ����壬�ȼ����¶Ƚϸߣ�����������ˮ����Һ����־�Ĥʱ�������¶ȼ������ȣ�Ȼ����ˡ�ϴ�ӣ��õ����壬ʵ��˳��Ϊdabace���ʴ�Ϊ��dabace��
(5)��ͼ���֪�����ٽ��£��ɵõ��ϴ�������壻�ʴ�Ϊ��C��
(6)A������ʽ�ζ�����Ϳ��ʿ��ʱ��Ϳ�ڻ����Ĵ�ͷ�ͻ�����С�ڵ��ڲ࣬��ac������A��ȷ��B���ζ�����ʱ����ƿ����Ҫ��ϴ����B����C����ת��Һ��ʱ������Ҫ������������C����D���ζ�ʱ��ͨ�������ֿ��������μ���Һ������ҡ����ƿ��ʹ��Һ��ͬһ������ת���۾��۲���ƿ����ɫ�ı仯�������D��ȷ��E���ζ�ǰ�ζ��ܼ����������ݣ��ζ�������������ݣ����ı�Һ�����ƫС�����õ������ʵ�����ĵ�С����E��ȷ������ʱ���۾������밼Һ�����ʹ���ƽ��������ȷ��0.01mL������Ϊ20.60mL���ʴ�Ϊ��ADE��20.60��

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�����Ŀ����ˮ����þ��MgSO4��7H2O����ӡȾ����ֽ��ҽҩ�ȹ�ҵ������Ҫ����;����þ������þ��������ɰ�ķ���������Ҫ�ɷ���MgCO3��������MgO��CaO��Fe2O3��FeO��MnO2��Al2O3��SiO2�����ʣ���ҵ������þ����ȡ��ˮ����þ�Ĺ���������ͼ��
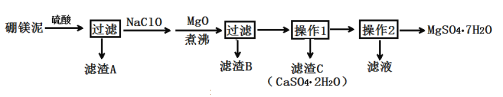
��֪����MnO2������ϡ���ᡣ
��CaSO4��MgSO4��7H2O�ڲ�ͬ�¶��µ��ܽ�ȣ�g���������±���ʾ��
�¶�/�� ���� | 10 | 30 | 40 | 50 | 60 |
CaSO4 | 0.19 | 0.21 | 0.21 | 0.21 | 0.19 |
MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | ���� |
��1����ʼ�õ��������������Ϊ70%���ܶ�Ϊ1.61g/cm3�����������Һ�����ʵ���Ũ��Ϊ___��
��2������A�г�������CaSO4��2H2O�⣬����___��
��3������MgO������е�Ŀ����___��
��4��������B����Ҫ�ɷ�ΪAl(OH)3��Fe(OH)3�������NaClO����������ԭ��Ӧ�����ӷ���ʽΪ___��
��5�������в���1Ϊ����Ũ�������ȹ��ˣ��������ɵõ�CaSO4��2H2O���ַ�ֹ___��
��6����ȡMgSO4��7H2O�IJ���2Ϊ��___��___������ϴ�ӡ�
��7����֪��ʼ��þ����Ʒ������Ϊag����ȡ��ˮ����þ������Ϊbg���ݴ��ܼ������þ����þԪ�صĺ��������ܣ���д������ʽ�������ܣ���˵�����ɡ�___���ܻ��ܣ�������ʽ�������ɣ�Ϊ___��