��Ŀ����
16����ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ��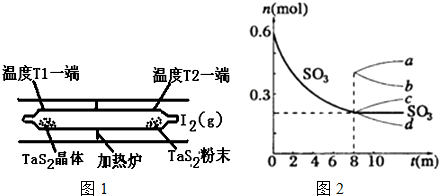
��1�����á���ѧ����ת�Ʒ����Ʊ�TaS2���壬�������·�Ӧ
TaS2��s��+2I2��g���TTaI4��g��+S2��g����H��0 �� I����
��ͼ1��ʾ����Ӧ�� I����ʯӢ��չ��н��У������¶�ΪT2��һ�˷���δ�ᴿ��TaS2��ĩ������I2��g����һ��ʱ������¶�ΪT1��һ�˵õ��˴�����TaS2���壬���¶�T1��T2���������������=������
��2������I2�������Կɲⶨ��������ĺ����������ǽ������е���ת��ΪH2SO3��Ȼ����һ��Ũ�ȵ�I2��Һ���еζ�������ָʾ��Ϊ������Һ���ζ���Ӧ�����ӷ���ʽΪH2SO3+I2+H2O=4H++SO42-+2I-��
��3��25��ʱ��H2SO3�THSO3-+H+�ĵ��볣��Ka=1��10-2mol/L������¶���NaHSO3��ˮ��ƽ�ⳣ��Kh=1.0��10-12������NaHSO3��Һ�м���������I2������Һ��$\frac{c��{H}_{2}S{O}_{3}��}{c��HS{O}_{3}��}$�������������С�����䡱����
��4����һ��2L���ܱ������У�������Ӧ��2SO3��g��?2SO2��g��+O2��g����H��0������SO3�ı仯��ͼ2ʾ��
����ʹ�÷�Ӧ�ķ�Ӧ����������ƽ�����淴Ӧ�����ƶ�����c��1�֣���
a������SO3�����Ũ�� b���ʵ������¶�
c��������䣬����ѹǿ d��ѡ���Ч����
�ڴ�8min��ѹ������Ϊ1L����SO3�ı仯����Ϊc��1�֣���
A��a B��b C��c D��d��
���� ��1��ͨ�������¶�T2�����ڷ�Ӧ������У�Ϊ���£��¶�T1�����ڷ�Ӧ������У�Ϊ���£�����T1��T2��
��2����ΪI2�������ۻ����ɫ�����Կ����õ�����Һ��ָʾ��������������ԭ��Ӧ��д��ѧ����ʽ��
��3������Ka=$\frac{c��HSO{\;}_{3}{\;}^{-}��c��H{\;}^{+}��}{c��H{\;}_{2}SO{\;}_{3}��}$��Kh=$\frac{[H{\;}_{2}SO{\;}_{3}]•K{\;}_{W}}{[HSO{\;}_{3}{\;}^{-}]•[H{\;}^{+}]}$���������ݽ��м��㣻
��4���پ�Ӱ�컯ѧ��Ӧ���ʺ�Ӱ��ƽ���ƶ������ؽǶ��ۺϿ��ǣ�
�ڸ���ѹǿ�Ի�ѧƽ���Ӱ�������
��� �⣺��1������������ʽ��֪�÷�ӦΪ���ȷ�Ӧ��ͨ�������¶�T2�����ڷ�Ӧ������У�Ϊ���£��¶�T1�����ڷ�Ӧ������У�Ϊ���£�����T1��T2��
�ʴ�Ϊ������
��2����ΪI2�������ۻ����ɫ�����Կ����õ�����Һ��ָʾ�����ζ��յ�ʱ��Һ����ɫ�仯Ϊ��ɫ��Ϊ��ɫ����Ӧ�����ӷ���ʽΪH2SO3+I2+H2O=4H++SO42-+2I-��
�ʴ�Ϊ��������Һ��H2SO3+I2+H2O=4H++SO42-+2I-��
��3��Ka=$\frac{c��HSO{\;}_{3}{\;}^{-}��c��H{\;}^{+}��}{c��H{\;}_{2}SO{\;}_{3}��}$��HSO3-+H2O?H2SO3+OH-��Kh=$\frac{[H{\;}_{2}SO{\;}_{3}]•K{\;}_{W}}{[HSO{\;}_{3}{\;}^{-}]•[H{\;}^{+}]}$=1.0��102��1.0��10-14=1.0��10-12������������I2ʱ��������ᣨ�����ᣩ������ǿ�ᣨ���ᡢ����ᣩ����Һ������ǿ��[H+]�������¶Ȳ��䣬Kh���䣬��$\frac{c��{H}_{2}S{O}_{3}��}{c��HS{O}_{3}��}$������
�ʴ�Ϊ��1.0��10-12������
��4����a������SO3�����Ũ�ȣ���Ӧ���ʼ������ʴ���
b������ƽ�������ƶ����ʴ���
c������ѹǿ����Ӧ���ʼӿ죬ƽ�������ƶ�������ȷ��
d��������Ӱ��ƽ���ƶ����ʴ���
��ѡc��
�������������С��ѹǿ����ƽ�����淴Ӧ�����ƶ���SO3�����ʵ������࣬�ʴ�Ϊ��c��
���� ������Ҫ���黯ѧƽ�ⳣ���ı���ʽ������㡢��Ӧ���ʡ�ƽ��ƽ���ƶ���ԭ������ͼ���������Ѷ��еȣ�

| A�� | ������������Һ�����ȣ������̪�Լ� | |
| B�� | ������������Һ�����ȣ�������ɫʯ���Լ� | |
| C�� | ������������Һ�����ȣ�������ĺ�ɫʯ����ֽ�����Թܿ� | |
| D�� | ������������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| A�� | �ƾ�������ɫ�Ľ��������۵����100�� | |
| B�� | �ƾ���ǿ��ԭ�ԣ�����CuSO4��Һ�����û���Ӧ����Cu | |
| C�� | ���������ڿ����м��������ɰ�ɫ���� | |
| D�� | �ƼغϽ��������³�Һ̬��������ԭ�ӷ�Ӧ�ѵĵ��ȼ� |
| A�� | �еĻ��Ϸ�Ӧ������������ԭ��Ӧ | |
| B�� | ���ֽⷴӦ��������������ԭ��Ӧ | |
| C�� | �û���Ӧһ����������ԭ��Ӧ | |
| D�� | CO��CuO��Ӧ�����û���Ӧ��������������ԭ��Ӧ |
| A�� | Na+��H+��AlO2-��C1- | B�� | ClO-��K+��OHһ��HSO3- | ||
| C�� | K+��Na+��SiO32-��SO42һ | D�� | K+��A13+��Cl-��HCO3- |
| A�� | ����ϡ���������ɫ���壬������ͨ�����ʯ��ˮ�У���Һ����ǣ�һ����CO32- | |
| B�� | �����Ȼ�����Һ�а�ɫ�����������ټ������ᣬ��������ʧ��һ����SO42- | |
| C�� | ����̼������Һ������ɫ�������ټ��������ɫ������ʧ��һ����Ba2+ | |
| D�� | ij��Һ�м�������������Һ������ɫ������һ����Cu2+ |

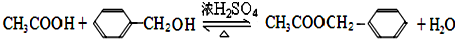 ��
�� ��
�� ��
��