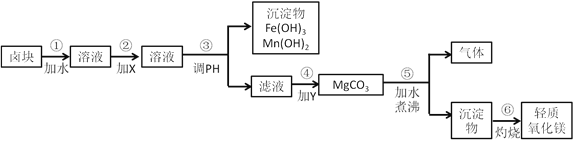
��Ŀ����
����Ŀ�����ײ����ij��A����Է�������Ϊ70���ش��������⣺
��1������������A�����������ϣ��������ʵ���һ�������ȼ����������������ȵ���(�����)_______��
a��C2H4b��C3H8c��C6H6d��C4H6O
��2������AΪ���������Ϻ���������������ֶΣ���ʾ�÷��Ӻ���3��-CH3��
��A�Ľṹ��ʽΪ__________��
������A��Br2��CCl4��Һ��Ӧ����B��B��NaOH�Ĵ���Һ���ȿɵõ�D��D����������ԭ�ӡ�����ʾ��̼ԭ����������̼̼˫���Ľṹ���ȶ�������д��D�Ľṹ��ʽ______��D�ܷ����ۺϷ�Ӧ���Ҿۺϲ�����������̼̼˫����д��D����E�ķ�Ӧ����ʽ______________��
��B������NaOHˮ��Һ��ȫ��Ӧ�������л���F���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3�����˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ6��:2��1��1����A������Ϊ____��
��4����A����ʹ��ˮ��ɫ������һ�ȴ���ֻ��һ�֣���A�Ľṹ��ʽΪ_________��
���𰸡� c (CH3)2C=CHCH3 CH2=CCH3CH=CH2 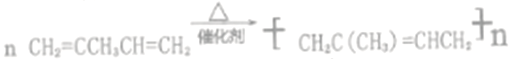 (CH3)2CBrCHBrCH3+2NaOH
(CH3)2CBrCHBrCH3+2NaOH![]() (CH3)2C(OH)CH(OH)CH3+2NaBr 3-��-1-��ϩ
(CH3)2C(OH)CH(OH)CH3+2NaBr 3-��-1-��ϩ ![]()
�����������ײ����ij��A����Է�������Ϊ70������70/14=5����A�ķ���ʽΪC5H10��
��1�������л���ȼ��ͨʽCxHyOz��(x+![]() ��
��![]() )O2
)O2![]() xCO2��
xCO2��![]() H2O��֪�����ʵ���һ�������ȼ����������������ȣ���һ������(x+
H2O��֪�����ʵ���һ�������ȼ����������������ȣ���һ������(x+![]() ��
��![]() )�Ƕ�ֵ��A��D��(x+
)�Ƕ�ֵ��A��D��(x+![]() ��
��![]() )�ֱ���3��5��7.5��5������A��(x+
)�ֱ���3��5��7.5��5������A��(x+![]() ��
��![]() )��7.5����ѡc����2��������AΪ���������A��ϩ�������Ϻ���������������ֶΣ���ʾ�÷��Ӻ���3��-CH3����A�Ľṹ��ʽΪ(CH3)2C=CHCH3��������A��Br2��CCl4��Һ��Ӧ����B��B���ṹʽΪ(CH3)2CBrCHBrCH3��B��NaOH�Ĵ���Һ���ȿɵõ�D���÷�Ӧ��±��������ȥ��Ӧ��D����������ԭ�����D�Ľṹ��ʽWΪCH2=C(CH3)CH=CH2��D�ܷ����ۺϷ�Ӧ���Ҿۺϲ�����������̼̼˫������D����E�ķ�Ӧ����ʽΪ
)��7.5����ѡc����2��������AΪ���������A��ϩ�������Ϻ���������������ֶΣ���ʾ�÷��Ӻ���3��-CH3����A�Ľṹ��ʽΪ(CH3)2C=CHCH3��������A��Br2��CCl4��Һ��Ӧ����B��B���ṹʽΪ(CH3)2CBrCHBrCH3��B��NaOH�Ĵ���Һ���ȿɵõ�D���÷�Ӧ��±��������ȥ��Ӧ��D����������ԭ�����D�Ľṹ��ʽWΪCH2=C(CH3)CH=CH2��D�ܷ����ۺϷ�Ӧ���Ҿۺϲ�����������̼̼˫������D����E�ķ�Ӧ����ʽΪ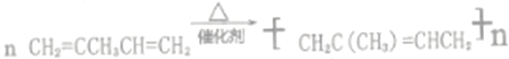 ����B������NaOHˮ��Һ��ȫ��Ӧ�������л���F��F�Ƕ�Ԫ�����÷�Ӧ�Ļ�ѧ����ʽΪ(CH3)2CBrCHBrCH3+2NaOH
����B������NaOHˮ��Һ��ȫ��Ӧ�������л���F��F�Ƕ�Ԫ�����÷�Ӧ�Ļ�ѧ����ʽΪ(CH3)2CBrCHBrCH3+2NaOH![]() (CH3)2C(OH)CH(OH)CH3+2NaBr����3�����˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ6��2��1��1����A�Ľṹ��ʽΪCH2��CHCH(CH3)2��������Ϊ3-��-1-��ϩ����4����A����ʹ��ˮ��ɫ�����A�ǻ�����������һ�ȴ���ֻ��һ�֣�����������ԭ����ȫ��ͬ����A�Ľṹ��ʽΪ
(CH3)2C(OH)CH(OH)CH3+2NaBr����3�����˴Ź���������ʾ����A�����鲻ͬ�ķ壬�������Ϊ6��2��1��1����A�Ľṹ��ʽΪCH2��CHCH(CH3)2��������Ϊ3-��-1-��ϩ����4����A����ʹ��ˮ��ɫ�����A�ǻ�����������һ�ȴ���ֻ��һ�֣�����������ԭ����ȫ��ͬ����A�Ľṹ��ʽΪ![]() ��
��

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�����Ŀ���±�1�dz����¼�������ĵ���ƽ��������Ka��������ĵ���ƽ�ⳣ����Kb������2ʱ�����¼����ѣ���������ܶȻ�������Kap��
��1 | |
���� | ���볣����Ka��Kb�� |
CH3COOH | 1.8��10-5 |
HIO3 | 1.7��10-1 |
HCN | 5��10-10 |
HClO | 3��10-8 |
NH3H2O | 1.8��10-5 |
��2 | |
�ѣ������� | �ܶȻ�������Ksp�� |
BaSO4 | 1��10-10 |
BaCO3 | 2.6��10-9 |
��ش���������:
(1)�����������У�������ǿ����______________(�û�ѧʽ��ʾ)��������ʹ������Һ��CH3COOH�ĵ�����������볣������IJ�����______(�����)��
A.�����¶� B.��ˮϡ�� C.��������CH3COONa���� D.������������
(2)HCOONH4��ˮ��Һ��_______(ѡ����ԡ��������ԡ��������ԡ�)��д��HCOONH4ˮ������ӷ�Ӧ����ʽ____________��
(3)���ʵ���1��1��NaCN��HCN�Ļ����Һ����pH>7������Һ�����ӵ�Ũ�ȴӴ�С����Ϊ___��
(4)��ҵ�г���BaSO4ת��ΪBaCO3���ٽ����Ƴɸ��ֿ����Եı���(��:BaCl2)�������������ñ��͵Ĵ�����Һ����BaSO4��ĩ�������ϲ��䴿����BaSO4ת��ΪBaCO3������������BaSO4����Һ���ڸ�����Һ�мӴ����ĩ�����Ͻ��裬���� SO42-���ʵ���Ũ�ȴﵽ0.05mol��L-1�����ʱ��Һ��CO32-���ʵ���Ũ��Ӧ_____________mol��L-1��