��Ŀ����
ͬһ���Ʊ�װ�ÿ�������ȡ��ͬ�����壬��ֻ����ͼװ����ȡ���壬��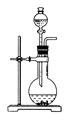
(1)�����±��ո�����������ҩƷ��
| �������� | ҩƷ | |
| ��Һ©���� | ��ƿ�� | |
| O2 | | MnO2 |
| H2 | ϡH2SO4 | |
| NH3 | | CaO |
| NO | ϡHNO3 | Cu |
| CO2 | ϡH2SO4 | ʯ��ʯ |
| C2H4 | C2H5OH | ϡH2SO4 |
| C2H2 | | CaC2 |
(2)д������ҩƷ�Ʊ�O2�Ļ�ѧ����ʽ ��
(3)д������ҩƷ�Ʊ�C2H2�Ļ�ѧ����ʽ ��
(4)�뻭������NH3��װ��ͼ�����������������������
(5)���и�ȫҩƷ����Ŀ�У����������Ʊ���Ӧ�������(�ɲ�����)��
������ ������ ��
������ ������ ��
������ ������ ��
(1)H2O2��п����Ũ��ˮ��H2O
(2)2H2O2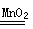 2H2O��O2��
2H2O��O2��
(3)CaC2��2H2O�D��C2H2����Ca(OH)2
(4)��ͼ��ʾ��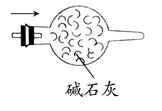
(5)��CO2��ϡ������ʯ��ʯ��Ӧ���ɵ�CaSO4�ܣ�������ʯ��ʯ���棬��ֹ��ϡ������ʯ��ʯ�Ľ�һ����Ӧ ��C2H4��C2H5OH������Ũ����������¼��ȣ���Ҫ�����¶���170��ʱ��������C2H4��
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����ͼ��ʾװ�ý���ʵ�飬��Һ��A��μ��뵽����B�У�����������ȷ����(����)
| A����AΪŨ���ᣬBΪMnO2��C��ʢƷ����Һ����C����Һ��ɫ�� |
| B����AΪ���ᣬBΪ���ǣ�C��ʢ����ʯ��ˮ����C����Һ����ǡ� |
| C��ʵ������D��Ҫ��ϴ�������á� |
| D����AΪŨ��ˮ��BΪ��ʯ�ң�C��AlCl3��Һ����C���Ȳ�����ɫ������������ܽ⡣ |
�������и����е������Ʊ����ռ�������Ӧ�����壬�ܲ�����ͼװ�õ���
��Ũ��ˮ����NaOH��NH3 �ڴ���ʯ��ϡ������CO2
�۹���������Һ�Ͷ���������O2��ϡ�����ͭƬ��NO
��Ũ����Ͷ���������Cl2 ��ʯ��ˮ��C2H2
|
����ʵ����ƻ�ʵ������У���ȷ����
| A��ͭ��Ũ���ᷴӦ��NO2���ɲ�����ͼװ�� |
| B������������Ҵ���Һ���÷�Һ©�����з��� |
| C��ϡ�����п����Ӧ��ȡ������������������ͭ�Լӿ췴Ӧ���� |
| D������Na2CO3��Һ��NaHCO3��Һ���ֱ���������Һ�μӳ���ʯ��ˮ������ |
���ᡢ��������Ტ��Ϊ��ҵ�ϵġ������ᡱ��ʵ������Ҳ������������ȡijЩ���壬��ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լ���װ�á��гֺ;���װ�ã������ô�װ�úͱ����ṩ�IJ�������������ʵ�顣
| ��� | a�е����� | b�е����� | c���ռ������� | d�е����� |
| �� | Ũ���� | Na2SO3 | �� | �� |
| �� | Ũ���� | Cu | �� | NaOH |
| �� | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
��1�������Т٢ڢ��������ʷֱ���__________��__________��________���ѧʽ����
��2��ʵ��������������۵����ӷ�Ӧ����ʽΪ________________________��ijͬѧ��Ϊ��Ũ�����Ϊϡ���ᣬ���������ñ���װ����ȡNO���壬��ͬѧ�����________�����ȷ������ȷ������
��3����֪ʵ��������������Ļ�ѧ����ʽΪ4HCl��Ũ��+MnO2
 MnCl2+ Cl2��+2H2O���ڸ÷�Ӧ��ÿ����22.4L����״���£�������������HCl�����ʵ���Ϊ__________��ת�Ƶ��ӵ����ʵ���Ϊ________��dװ���з�����Ӧ�����ӷ���ʽΪ_________________��ʵ���һ������ø�����غ�Ũ���ᷴӦ��ȡ��������ɲ���ƽ�÷�Ӧ�����ӷ���ʽ��
MnCl2+ Cl2��+2H2O���ڸ÷�Ӧ��ÿ����22.4L����״���£�������������HCl�����ʵ���Ϊ__________��ת�Ƶ��ӵ����ʵ���Ϊ________��dװ���з�����Ӧ�����ӷ���ʽΪ_________________��ʵ���һ������ø�����غ�Ũ���ᷴӦ��ȡ��������ɲ���ƽ�÷�Ӧ�����ӷ���ʽ��____ MnO4��+ ____ H+ + ____ Cl��
 ____ Mn2+ + ____ Cl2��+ ____ _________
____ Mn2+ + ____ Cl2��+ ____ _________ ��ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1~10nm֮�䣩����ͼ��ʾA~EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⣮
��1��ʵ�����Ʊ�NH3�ķ�Ӧ����ʽ��_______________________________��
��2��ʵ������ȡ���ռ������NH3����ѡ����������װ�õĽӿ�����˳���ǣ�ѡ����ĸ����a�� �� �� �� ��h��
��3����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ��������� ����д��������̼��Ƶ����ӷ���ʽ ��
��4����ŨCaCl2��Һ��NH3������ȷ������£�CO2�����������ᵼ������̼��Ʋ����½�����CO2������Һ�д������ڵ������У�������������ʵĵ��������ˮ��������������ӣ�________________,
��5��ȡ��Ӧ��ȥ����CaCO3����Һ�ֱ�������ʵ�飬����ʵ���жϺ������ǣ�_________��
| A���μ�����Na2CO3��Һ�����г���˵��CO2һ������ |
| B���μ��������ᣬ�������ݣ�CO2һ������ |
| C��������ҺpH��������7��CO2һ�������� |
| D���μ�����BaCl2��Һ����������CO2һ��û�й��� |
��ҵ�Ͽ���ʳ�κ�ʯ��ʯΪ��Ҫԭ�ϣ�����ͬ�ķ������������ش��������⣺
��1��¬����������ʳ�Ρ�ʯ��ʯ��Ũ���ᡢ��̿Ϊԭ�ϣ��ڸ����½������գ��ٽ�ȡ���ᾧ���Ƶô��
��ʳ�κ�Ũ���ᷴӦ�Ļ�ѧ����ʽΪ___________��
�������ƺͽ�̿��ʯ��ʯ��Ӧ�Ļ�ѧ����ʽΪ_________����֪����֮һΪCaS����
��2������Ĺ�������ͼ��ʾ���õ���̼�����ƾ��������ɴ��
| | |
| |  |
��װ�����з�����Ӧ�Ļ�ѧ����ʽΪ_______��

��3�������Ƽ����ĸĽ������ŵ���______________��
��4��������Ϊ̼�������̼�����ƵĻ�ѧ�������ƣ���Ҳ���ð�����Ȼ��غ�ʯ��ʯΪԭ����̼��ء�������ͼ���ܽ�ȣ�S�����¶ȱ仯���ߣ�����˵���Ƿ���У�__________��
��̼���ƣ�Na2CO3��3H2O2�����й���˫��ˮ���׳ƣ��þ������Na2CO3��H2O2��˫�����ʣ�������Ӧ����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������У���̼���Ƶ�ij������������ͼ��ʾ��
��֪��2Na2CO3��3H2O2��2Na2CO3��3H2O ��H��0���ش��������⣺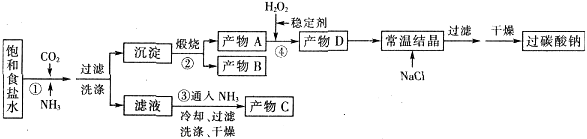
��1���������ʿ�ʹ��̼���ƽϿ�ʧЧ���ǣ�����ţ�_________��
| A��FeCl3��Һ | B��H2S | C��ϡ���� | D��NaHCO3��Һ |
��3�������������У���Ӧǰ��H2O2�м����ȶ�����������____________________��
��4�������������п�ѭ��ʹ�õ�������______________________________���ѧʽ����
��5��������̼���Ƶ���������©��һ����������ò�Ʒ����ƫ�ͣ�������ò���������___________��
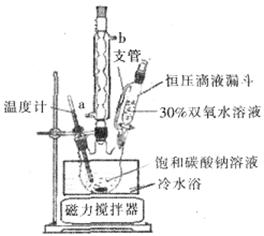
��6��ʵ����������ͼװ���Ʊ���̼���ƣ���װ���к�ѹ��Һ©����֧�ܵ�������_______��������Ӧ��__________����ˮ��
��7����ʵ��ⶨ��Ӧ�¶ȶԲ����Ӱ�����±��������±����ݣ�����Ϊ��Ӧ��ѵ��¶�ѡ��ķ�Χ��_______________��
| T/�� | �������ٷֺ��� | ���� |
| 5��10 | 13.94 | 85.49 |
| 10��15 | 14.02 | 85.78 |
| 15��20 | 15.05 | 88.38 |
| 20��25 | 14.46 | 83.01 |
 ��
�� 