��Ŀ����
��ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1~10nm֮�䣩����ͼ��ʾA~EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⣮
��1��ʵ�����Ʊ�NH3�ķ�Ӧ����ʽ��_______________________________��
��2��ʵ������ȡ���ռ������NH3����ѡ����������װ�õĽӿ�����˳���ǣ�ѡ����ĸ����a�� �� �� �� ��h��
��3����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ��������� ����д��������̼��Ƶ����ӷ���ʽ ��
��4����ŨCaCl2��Һ��NH3������ȷ������£�CO2�����������ᵼ������̼��Ʋ����½�����CO2������Һ�д������ڵ������У�������������ʵĵ��������ˮ��������������ӣ�________________,
��5��ȡ��Ӧ��ȥ����CaCO3����Һ�ֱ�������ʵ�飬����ʵ���жϺ������ǣ�_________��
| A���μ�����Na2CO3��Һ�����г���˵��CO2һ������ |
| B���μ��������ᣬ�������ݣ�CO2һ������ |
| C��������ҺpH��������7��CO2һ�������� |
| D���μ�����BaCl2��Һ����������CO2һ��û�й��� |
��1��Ca(OH)2+2NH4Cl CaCl2+2NH3��+2H2O��2��d,e,g,f
CaCl2+2NH3��+2H2O��2��d,e,g,f
��3��NH3 Ca2++2NH3+H2O+CO2=CaCO3��+2NH4+
��4��Ca2+ HCO3- NH4+ Cl- ��5��B ��6��ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·�����������������ǡ�
���������������1��ʵ�����ư��������Ȼ�狀��������Ƽ����Ƶã������Ȼ��ơ�������ˮ����Ӧ����ʽΪCa(OH)2+2NH4Cl CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��2��ʵ�����Ʊ��������������װ������Ϊ������װ�á���������װ�á��ռ�װ�ú�β������װ�á�ʵ������ȡ�������ù��塢���������װ�ã�ӦѡAΪ����װ�ã��������к���ˮ����������ˮ�Լ��ԣ�Ӧѡ���ʯ�ҵȹ�������������ˮ��������������һ��ʢ���ڸ���ܻ�U�ι��У���ѡC��������������ˮ�����ܶȱȿ���С��Ӧ���������ſ������ռ�����ѡE�������д̼�����ζ���ж�������ֱ���ŷŵ������У�������������ˮ������β������Ӧ���÷�ֹ����װ�ã���ѡD��ע�����ܵ�ʹ��ԭ���Ǵ�ڽ�С�ڳ�����������װ�õĽӿ�����˳����d��e��g��f��
��3��������������ˮ��������̼��������ˮ������Ӧ��ͨ�백������������ˮ���ɰ�ˮ����Һ�ʼ��ԣ��������ո���Ķ�����̼���������̼��ƵIJ�����������̼�ܺͰ�ˮ��Ӧ����̼��泥�̼��狀��Ȼ��Ʒ������ֽⷴӦ����̼��ƺ��Ȼ�泥����ӷ���ʽΪ��Ca2++2NH3+H2O+CO2=CaCO3��+2NH4+��
��4����ͨ�����CO2�����ķ�ӦΪ��CaCl2+CO2+2NH3+H2O=CaCO3��+2NH4Cl��
CO2+ CaCO3+ H2O =2Ca(HCO3)2����Һ�д������ڵ�������Ca2+ ��HCO3- ��NH4+�� Cl-��
��5��CO2������Һ�к���Ca2+ ��HCO3- ��NH4+�� Cl-��CO2���㣬��Һ�к���Ca2+ ��NH4+�� Cl-�Ͱ�ˮ��A���μ�����Na2CO3��Һ���г���CO2���ܲ���Ҳ���ܹ���������B���μ��������ᣬ�������ݣ�CO2һ����������ȷ��C��CO2������������Һ�������Լ��ԣ�pH����7������D��CO2�����������μ�����BaCl2��Һ�����������ɣ�����
��6��̼�����Ʒ�������Ϊ��������������ɢ��ˮ�����γɽ��壬���ý���Ķ����ЧӦ�жϣ���Ϊ��ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·�����������������ǡ�
���㣺���鰱����ʵ�����Ʒ�����°��Ƽԭ�������Ӽ���ͽ��������

ʵ���������и������壬�������巢��װ����ͬ����
| A��H2��CO2 | B��H2��O2 | C��Cl2��CO2 | D��Cl2��H2 |
����װ����ʾ��ʵ���У��ܴﵽʵ��Ŀ�ĵ���
| A����ȥCl2�е�HCl | B����������������� |
| C��ʵ�����ư��� | D���������еĵ�;ƾ� |
ͬһ���Ʊ�װ�ÿ�������ȡ��ͬ�����壬��ֻ����ͼװ����ȡ���壬��
(1)�����±��ո�����������ҩƷ��
| �������� | ҩƷ | |
| ��Һ©���� | ��ƿ�� | |
| O2 | | MnO2 |
| H2 | ϡH2SO4 | |
| NH3 | | CaO |
| NO | ϡHNO3 | Cu |
| CO2 | ϡH2SO4 | ʯ��ʯ |
| C2H4 | C2H5OH | ϡH2SO4 |
| C2H2 | | CaC2 |
(2)д������ҩƷ�Ʊ�O2�Ļ�ѧ����ʽ ��
(3)д������ҩƷ�Ʊ�C2H2�Ļ�ѧ����ʽ ��
(4)�뻭������NH3��װ��ͼ�����������������������
(5)���и�ȫҩƷ����Ŀ�У����������Ʊ���Ӧ�������(�ɲ�����)��
������ ������ ��
������ ������ ��
������ ������ ��
��ѧС���������������������װ��(����ͼ)���û������Ʊ�����ϩ��
��֪��
| | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |

(1)�Ʊ���Ʒ
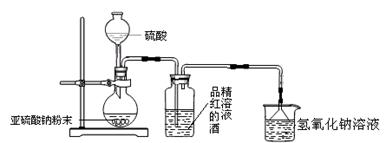
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ����ֹ���У���������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�ٵ���B���˵�������е�������________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��________��(��ϡ����¡�)����Һ����________(������)ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ

���ٽ�����ϩ����ͼװ��������ȴˮ��________�ڽ���(�g����f��)������ʱҪ������ʯ�ң���Ŀ����_____________________________________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________��
A������ʱ��70 �濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������________��
A���ֱ�������Ը��������Һ
B���ֱ�����ý�����
C���ֱ�ⶨ�е�


 N2��+2O2��+Cl2��+4H2O���ֿ��ṩ�����Լ���
N2��+2O2��+Cl2��+4H2O���ֿ��ṩ�����Լ���

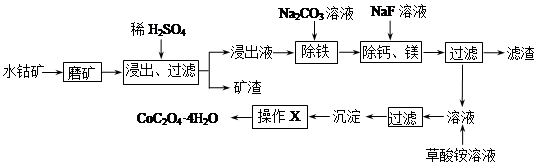

 ��
�� CoO2+LiC6��д���õ�طŵ�ʱ��������Ӧʽ ��
CoO2+LiC6��д���õ�طŵ�ʱ��������Ӧʽ ��
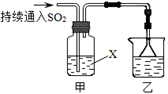
 Ca2��+
Ca2��+ ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ��Ļ��������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ��Ļ��������������£�